Abstract
Eurycoma longifolia plant, the so called Tongkat Ali in Malaysia, is a well grown prominent tree in all Southeast Asia. It is well known among traditional medicine practitioners as a curative plant for many diseases and health conditions. The major quassinoid from the plant is eurycomanone, which exhibits many prominent effects on various cancer cell lines. Numerous studies have shown that eurycomanone inhibits cancerous cell growth and encourages cell death both in vitro and in vivo test. Even though analyses of safety and toxicity have been conducted, there is still a substantial knowledge barrier when it comes to providing a scientific foundation for the molecular mechanism as well as intervention strategy in the living people cancer cell. In a way to offer adequate baseline data for future investigations based on molecular mechanism and intervention, the present work seeks to review the researches conducted to date on this herbal plant.
Key words: Eurycoma longifolia, Cancer, Molecular mechanism, Pharmacological studies
Introduction
In reference to the World Health Organization (WHO) specifically, complementary treatment is known as “the overall of the knowledge, general skills, and practice based on the theories, trust, and experiences to different cultures, whether or not, used in the preservation of general health as well as in the diagnosis, prevention, improving or remedies of physical and mental diseases”.1 According to the WHO, herbal or plant preparations, herbal ingredients, and any finished herbal products that contain plants, other plant materials, or mixtures as a bioactive compound are all considered complementary medicine.2 This refers to the extensive historical usage of herbal or plant-based medicines or their supplementary use. The usage of these medications are indeed wellestablished, well accepted for their effectiveness, and are acknowledged by national health organizations.2 According to the WHO’s estimates, 85% of people worldwide rely to some extent on herbal remedies for their primary healthcare.3 It is well-established fact that complementary medications, along with traditional and alternative medicines, are used in our healthcare system. This is a compact- documented data that the complementary medicines as well as traditional and complimentary medicines have a well-established usage in our healthcare. One of the popular and commonly available herbs in Southeast Asia and Indo China is Eurycoma longifolia which is from Simaroubaceae family. The roots are widely used for many diseases and medical illness in many Asian regions. Apart from it, recently, E. longifolia plant has also demonstrated excellent examples in herbal therapy as supplementary and alternative medicine in the West. Also known as Tongkat Ali in Malaysia, it is aphrodisiac in nature and has a popular reputation locally and globally.4,5 The aqueous extract and decoction made from its root have been shown to enhance fertility, sexual activity and possess anti-aging properties.6 E. longifolia extract can also be utilized as complementary remedies to avoid and address angiogenesis, as well as showing anti-cancer activities on different solid cancer cells, including lung, breast and cervical cancers.7-9 The anti-malarial, anti-inflammatory, anti-oxidant and anti-microbial are the most common activities exerted by this plant.10 Studies have revealed that E. longifolia extracts mainly consist of flavonoids, alkaloids, phenolics, saponins, tannins, triterpenes, and quassinoids such as eurycomanone, eurycomalactone and eurycomanol that are contributing to these biologic activities.11 Eurycomanone is the most prominent compound isolated from E. longifolia Jack for the treatment of cancer in which its cytotoxicity has been documented against different cancer cell lines, and apoptosis has been shown as the vital mechanism involved in cellular toxicity.12 The roots of E. longifolia has been well documented for its medicinal usage such as anti-inflammatory, antimalarial, and aphrodisiac activity. The ethno biology information of E. longifolia has been continuously and widely applied by indigenous people till to date. In particular, the plant roots are believed to have higher therapeutic effects than other parts of the plant. Recent scientific studies have also proven the remarkable pharmacological properties of the plant, mostly the plant roots. This can be seen from the previous reported findings which have been gathered and systematically compiled in Table 1.13-22 The chemical structures of the reported compounds from E. longifolia extracts are presented in Figure 1. The data reveals that scientific studies on the main pharmacological properties of the plant have been extensively carried out since 1980s. The promising results have also boosted the application of the plant extracts and the acceptance of public using E. longifolia in health promoting interest. The technical evidences explained that the ethnopharmacological applications were mainly contributed by phytochemicals in the plant roots. The major classes of phytochemicals, particularly canthin-6-one alkaloids quassinoids, β-carboline alkaloids, biphenylneolignans, squalene derivatives and have been reported to possess the pharmacological significance. 23 Most of the reported compounds in E. longifolia belong to the group of quassinoids or degraded triterpenes. One of the commonly known quassinoids is eurycomanone which is also a C 20-type quassinoids and the marker compound of the plant. Hence, this review article aims to compile studies related to E. longifolia and its major quassinoid compound (i.e. eurycomanone) for their anti-cancer activities regarding its molecular mechanism and intervention updates.
Materials and Methods
The comprehensive and pertinent clinical trials, review articles, translational papers, and research papers on precision oncology and immuno-oncology were chosen. This paper includes 48 literature reviews and database searches using keywords syntax from Pubmed, Google scholar, Science direct and other relevant publications article published between 2005 until 2022. Based on their uniqueness and possible therapeutic usefulness, papers were ranked and chosen. Inclusion criteria were chosen based on pharmacological activities reported in vitro and in vivo while we excluded papers with general review on Tongkat Ali especially not related to cancer.
Pharmacological activities
E. longifolia also has antiproliferative and cytotoxic effects on a number of human cancers cell lines, as well as solid tumours such lung, breast, and cervical malignancies. These effects are in addition to its influence on fertility. In general, it is crucial to consider the cytotoxic effects of novel drug entities and treatments before examining their pharmacological efficacy. Once effective cytotoxic effects have been established, cytotoxic effects (rate of cytotoxicity) are studied in vitro and in vivo to gauge and confirm their effectiveness against cancer.
Several components of E. longifolia extract have been worked for cytotoxicity, and some of them have shown to have anti-proliferative properties.14 Eurycomanone is a carcinogenic bioactive component abundantly identified in E. longifolia Jack that causes cytotoxicity in a multiple epithelial cell culture lines. In one reported research work, eurycomanone demonstrated antiproliferative activity against several malignant cell lines (i.e. Hela, HM3KO, Caov-3, MCF-7, and Hep G2) and were proven to exhibit cytotoxic towards HeLa cells via apoptosis.24,25 The compound was also shown to be generally harmless on cell lines which are non-cancerous. (i.e. Vero, MDBK). This shows that the compound may be a safer alternative as an anticancer to reduce the systemic side effects.
Table 1.
Summary of chemical entities of Eurycoma longifolia and the pharmacological activities.
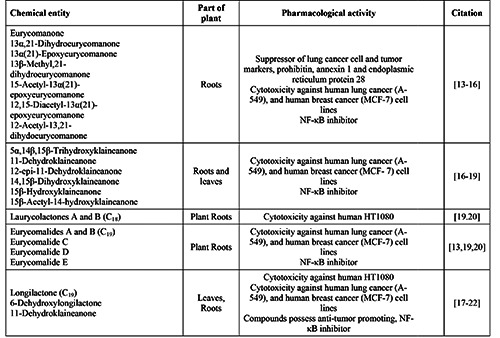
The pharmacological activity of E. longifolia extract, particularly eurycomanone, on inhibition of cancer cells proliferation has been attracting interest in this few decades. This activity has been further investigated on different cancer cell lines for their molecular mechanisms which will be reviewed in the next section.
Molecular mechanisms of ant proliferative and cytotoxic activities
Different constituents from Tongkat Ali have been tested and shown promising anti-proliferative and cytotoxic effects.26 Antiproliferative activities of E. Longifolia are mainly contributed by two major quassinoids, eurycomanone and longilactone, which induce tumour cell death via different pathways, as shown in Figure 2 below.
Initially, eurycomanone is reported to induce cell death via down regulating of Bcl-2 protein together with cleavage of protein caspase-7 and PARP-1.27 However, later study has shown that the cell death was caused by the increase of the proteins bax and p53, as well as the inhibition of the protein Bcl-2, in a number of cancer cells.28 Further studies showed that a major reduction in the expression upon treatment with eurycomanone, it was discovered that tumour markers like heterogeneous nuclear ribonucleoprotein (A2/B1 (hnRNP-A2/B1), the tumour inhibitor protein p53, and other sub genes like endoplasmic reticulum protein-28 (ERp28), prohibitin (PHB), and annexin-1 (ANX-1) were altered in A549 lung cancer cell culture lines (64). Apart from that, longilactone showed its anti-cancer action via the activation of caspase-7 and 8, and Poly (ADP-ribose) polymerase but caspase-9 not affected, and both of Bcl-2 and Bax proteins which remained unchanged.13
Figure 1.
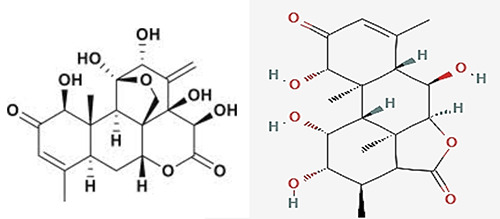
Chemical structures of eurycomanone and longilactone (Biotropics Malaysia Berhad (Biotropics).
Figure 2.
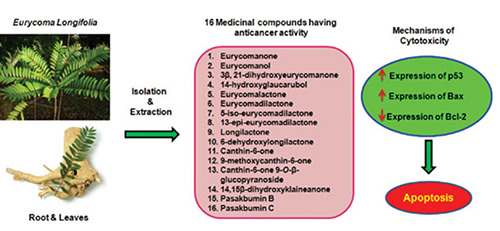
Mechanism of action of eurycomanone and longilactone on cell apoptosis.
A study by Tong and co-workers has tested the cytotoxic potential of various methanolic extracts of E. longifolia root. Quassinoid-rich fraction which is partially purified was tested against K-562 (human lymphoblastoid) cells with imatinib as a positive control. After 48 hours treatment, the percentage showed inhibition in cell growth and induced the cell apoptosis. The outcome also showed clearly the fraction induced cell death in K-562 cells in a specific p53 dependent cell growth. However, it reported the intervention mechanism action by p53 was unclear and it might involve a few other modes of action. Moreover, it was shown that osteoclast cell development and cell differentiation were inhibited. The molecular mechanism study further showed that down regulation of prominent receptor activator of nuclear factor-kappa-Β ligand (RANKL) induced TRAP tartrate-resistant acid phosphatase(TRAP) activity and expression of MMP-9, cathepsin- K, NFATc1 and release of superoxide chemicals and enhanced superoxide chemicals dismutase activity in the treated cancer cells.14
Many clinical investigations have been carried out to evaluate the benefit of pharmacology target of different anti-angiogenic substances. Inhibiting endothelial cell’s function, which is believed to be the building gene of blood vessels, seems to be main mechanism of these agents. E. longifolia can be utilised as complementary remedies to prevent this angiogenesis related problems. In a separate study, a research work has been done on the evaluation of purified quassinoid-rich fraction of E. longifolia root extract in ex vivo and in vivo models.12,13 Results showed that the extract caused prominent suppression of micro vessels growth in aorta of rats. It also showed inhibition of neo vascularization in chick embryo on chorioallantoic membrane by 63.12%, which the vascularisation was also shown to be reduced in histological studies.29 The extract caused significant inhibition of cell differentiation, migration and proliferation in human umbilical vein endothelial cells segments. Further, HPLC analysis confirmed the chemical entity which is eurycomanone, 13α,30,31 -epoxyeurycomanone and eurycomanol were found as the major content in the extract. These outcome shows that the anti-angiogenic effect of E. longifolia may be due to its inhibition effect on endothelial cell growth, migration and proliferation which could be attributed to the high level of quassinoids. 32
Another study by Wong and co-workers, eurycomanone exhibited effects on various cancer cell lines that were antiproliferative. 32 Removal of eurycomanone did not stop the antiproliferative effects in which 30% of inhibition was still intact. Many parameters of lung cancer were reduced by the treatment of eurycomanone, which are heterogeneous type nuclear ribonucleoprotein (hnRNP) A2/B1, tumor-related genes including prohibitin (PHB), annexin 1 (ANX1) and endoplasmic reticulum type protein 28 (ERp28) and p53 tumour inhibitor protein.33 Every gene’s expression except one is PHB was not regulated post 72 hours after therapy (P is less than 0.05, T-test, n=9). This study suggests that quassinoid type eurycomanone is a vital therapeutic agent and exerts its activity in a dose concentration range from 5–20 mcg/mL.
Interestingly, eurycomanone also poses cytotoxic activity as reported in many research works. Cells that mainly showed general apoptosis response following treatment with eurycomanone are epithelial cells.34 A recent study shows that eurycomanone demonstrated anti-proliferative effect in many cancerous cells such as malignant breast cells (MCF 7), ovarian malignant cells (Caov 3), liver cancerous cells (Hep G2) and human malignant melanoma cell lines (HM3KO) and yet were not toxic to non-cancerous cell lines such as MDBK and VERO.35,36 The molecular mechanism of eurycomanone in HeLa cells was detected using TUNEL assays where fluorescence detection in nuclear region indicated the presence of DNA fragmentation. The treatment duration plays a vital role in this test by which the fluorescence became more prominent with a longer duration. For HeLa cells, staining with nuclear fluorochrome further confirmed the cell death mechanism which was similar to tamoxifen treatment.37
Drug candidates for anti-cancer should possess the capacity to restrain proliferation in tumour cells and induce cell death. As previously reported, bioactive compound such as eurycomanone has shown reduction in the persistence and growth of many cancerous cells such as HM3KO, HepG2, CaOv-3, MCF-7 and HeLa cells.38 Treatment on malignant cells with eurycomanone including (HeLa, CaOv, HepG2, MCF7, HM3KO) cell lines greatly decreases quantity of viable cells. It is also found that the eurycomanone is relatively benign to non-cancer cell lines (i.e. Vero, MDBK), which was in agreement with the report in previous work on the least cytotoxicity of eurycomanone on non-malignant related breast cancer cells (MCF 10A).39
Breast cancer
E. longifolia has shown inhibition into malignant breast cancer cell line proliferation (MCF 7). The possible molecular mode of action is modulation of Bcl-2 release by decreasing the amount of this protein. Studies on the biological mechanism of in E. longifolia supply the basic chemical information for their anti-proliferative impact at the cellular level. The platform IS giving more powerful chemopreventive and even chemotherapeutic active ingredients. 27 An interesting finding has been shown in the combination of eurycomanone and doxorubicin treatment against cancerous breast cell lines.40 The cytotoxic action of eurycomanone with doxorubicin was tested using colorimetric MTT assay, and the cell death activity was evaluated using flow cytometry against MCF-7 and T47D cells. Apoptosis or cell death was highly prevalent in MCF 7 cancer cells tested with eurycomanone itseld when compared to doxorubicin alone. While in T47D cells, doxorubicin alone showed more cell death than eurycomanone by itself, even though not statistically significant (P>0.05). Apoptosis was prominent in both malignant cancer cellular lines when eurycomanone together with doxorubicin were in combination in comparison to doxorubicin alone in both tests.
In another study, the efficacy of eurycomanone in inhibiting cell growth and its mechanism was studied on MCF 7 cell lines.41 Eurycomanone was tested in an increasing concentration manner and viability was measured via dye inclusion method using methylene blue. The results showed the anti-cytotoxic effect of the compound on MCF 7 cancer growth cells with inhibitory concentration (IC50) reading of 2.2±0.18 mcg/ml. Reduction in the number of cells suggested a cytotoxic effect which was later confirmed using TUNEL assay that the death of the cured cancer cells was via apoptosis when compared to the non-cured nucleus. Measurement by time indicated that cell death increased from 70% in 24 hours to more than 80% in 48 hours and subsequently to more than 85% after 72 hours. Nuclear staining method further showed fragmentation of nuclear cells and not necrosis. The reduced expression of BCL-2 at 2-hour post treatment was showed in Western blotting method. Further, pro-apoptotic protein which is Bax remained at a low level indicating possible shifting of the Bax to Bcl-2 which indirectly causes cell death. It was also suggested that the p53 expression was not affected. Few other parameters were functional such as procaspase-8 and procaspase-9. Caspase 8 was responsible for targeting Bid and caspase 7, which eventually spitted and inactivated PARP while caspase 9 targeted caspase 6 which divided and activated lamin. Both are important nuclear membrane proteins for normal cells to function and control of cell cycle.30
Cancer of prostate
The second common malignant disease in the world is prostate cancer. Few studies have reported the prostate cancer; which ic PC-3 cells are more susceptible to the cytotoxic effects of E. longifolia jack root extract thanks to an increase in apoptosis. It has been demonstrated that E. longifolia root extract increases the apoptotic activity proportionate to the concentration of the extract in PC-3 cells. Hence, a higher level of cell death has been recorde.42
Prostate cancer treatment with E. longifolia’s SQ40 quassinoid extract was also investigated. The anti-proliferative and anticancer effects of SQ40, which includes 40% (w/w) quassinoids, were examined in human prostate cancer. It demonstrated a potent, dose-dependent cytotoxicity effect against the LNCaP cell line for human prostate cancer. The chemical mechanism through which eurycomanone triggered cytotoxicity that led to apoptosis in cancer cells is primarily chromatin condensation.24,25
Nasopharyngeal cancer
Ethanol and dichloromethane root extracts of E. longifolia has anti-cancer properties and has been established against the nasopharyngeal carcinoma (ORL-115) cell line. The dichloromethane extract exhibited a lower IC50 value (42.6 μg/mL) compared to ethanol extract (73.72 μg/mL) after 72 hour, and the cytotoxic activity of both extracts was dose and time-dependent.43
Liver cancer
Another research finding indicated that eurycomanone has cytotoxic effect on liver cancer cells, HepG2 protein, and was reduced cytotoxic on non-cancer cells, including WLR 68 and Chang’s liver.32 Studies suggested that cell death was the main molecular mechanism of cell apoptosis in the eurycomanone treated liver cancer cells. The molecular mechanism includes chromatin condensation, DNA fragmentation and the appearance of cytotoxic bodies upon eurycomanone treatment. The upregulation of p53 tumour suppressor protein was found to be responsible for the eurycomanone cytotoxic effects. This was followed by an increase in protein called pro apoptotic, Bax protein and reduction of anti-apoptotic protein Bcl-2. Additionally cytochrome type C levels in cytosol were as well increased as a result of activation of cell death due to eurycomanone exposure.44
Lung cancer
Eurycomanone also showed strong anti-cancer activity against lung cancer cells. For an example, purified eurycomanone expressed apoptosis in selected cells in human lung cancer adenocarcinoma (A549) within a concentration ranging of 5-20 mcg/mL. Significant effect of eurycomanone was shown as lowest cell growth yet observed in a dose corresponding manner at 20 mcg/mL. Further molecular mechanism investigation revealed the expression of Bax, p53 protein, and Bcl 2 using Western blotting and immune staining test assays eventually decreases.45
Leukemia
Using human leukaemia cells, K-562, E. longifolia’s antileukemia properties have been documented. A significant growth inhibition and cell death were observed when K-562 cells were given treatment with E. longifolia fractions of (F3,F4A and TAF273). In vivo animal studies also proved the anti-leukemic effects of the fractions with reduced tumour volume and increasing numbers of apoptotic cells as compared to control groups.
Other cancers
Eurycomanone has also posed cytotoxic effect on Hela, HepG2, MCF 7, HM3KO and CaOv-3 cell lines with lesser toxicity against the normal cells (i.e. Vero, MDBK). Apoptosis was identified as the main mechanism of action of eurycomanone along with DNA fragmentation, apoptotic body formation and chromatin condensation.38 In another study, p53 up regulation of tumour suppressing protein was shown to be activated by eurycomanone which the mechanism involved in maximising pro apoptotic.9
Safety and toxicity
Despite E. longifolia has generally utilized around Malaysian’s traditional medicine for many years, researchers only began to give more attention to its safer dosage and toxicity profiling in the late 1990s. Tongkat Ali (or E. longifolia) quantities used with an average of (2.5 g•mL1) seem to have no negative effects on human spermatozoa in vitro, according to safety tests conducted thus far.33 Although in vivo evidence from Tambi and Kadir indicates that the plant extract were not harmful, at doses greater than 100 g•mL1, cytotoxic effects may occur if used uncontrolled.28 The orally used Lethal Dose 50 (LD50) of the alcoholic extract of E. longifolia in mice is between 1500-2000 mg/kg, whereas the oral LD50 of the aqueous extract form is more than 3000 mg/kg, according to a study by Satayavivad et al. No adverse effects on the offspring could be found in animal studies, including malformations, effects on weight of the body or the number of offspring. These authors also demonstrated that daily doses of 300 mg/kg of the aqueous extract and 200 mg/kg of the ethanolic extract were not harmful. More significant hepatotoxic effects in the rat were only observed at concentrations more than 1300 mg/kg body weight. It merely means that because the composition of the E. longifolia ethanolic, n-butanolic, and aqueous-based fractions varies, so do the LD50 values and daily effective dosages for each component. Since the LD50 value of the water-based fraction of E. longifolia is relatively high (>3000 mg/kg) compared to other fractions, it is important to take this into consideration when employing different fractions of E. longifolia and to properly reference the relevant range of LD50.33 In a rat model, Choudhary et al. looked at the toxicity of the standardised aqueous E. longifolia extract (Physta®) at the acute, subacute, and subchronic levels. E. longifolia was administered to female and male Wistar rats for 90 days at doses ranging from 250 mg/kg to 2000 mg/kg.41 Results unambiguously demonstrate that blood chemistry and haematological parameters have not changed significantly. Additionally, there were no histological abnormalities, and even in acute toxicity testing, the animal behaviour or death did not change. The Endocrine Society advises that any testosterone medication should be considered contraindicated in the case of prostate cancer (PCa).46 It also mentioned E. longifolia extract raises serum testosterone levels, there may be a danger risk associated with its use in older men because it could result in prostatic issues. However, a randomised double-blind, placebo-controlled clinical investigation by Ismail et al. We found no change in serum levels of prostate-specific antigen (PSA) between the verum and placebo groups. According to Li et al., no mutagenicity or clastogenicity was detected, and the LD50 (acute oral dose) for E. longifolia extract were greater than 6 g/kg b.w. After exposure paradigms of 0, 0.7, 1.3, and 2.1 g/kg body weight per day for 4 and 13 weeks, respectively, no adverse effects attributed to the test substance were seen in terms of weight of the body, haematological, serum biochemistry, urinalysis, macropathology, or histology. Partial thromboplastin time, Prothrombin time, creatinine, blood urea nitrogen(bun), aspartate aminotransferase(amt), lactate dehydrogenase(ld), and cholesterol levels were all significantly lower after the treatment, especially in men (P 0.05). Up to 1.2 g/day per adult was the calculated and acceptable daily intake (ADI) for E. longifolia extract.47 For managing safety and product development, this data is helpful. TAF273-treated pregnant female rats showed no toxic symptoms, and their pregnancies were healthy with no abnormalities in the foetus. In rat reproductive toxicity and teratology experiments, TAF273 was given at a dose of 100 mg/kg per day, which is almost 10 times lower than the LD50 value. No adverse effects were noted. According to the authors, any human dose created by converting rat doses of 100 mg/kg/day or less may be deemed safer for use in future clinical research. According to the Food and Drug Administration, normalising to BSA, which is frequently expressed in mg/m2, is the only way to appropriately extrapolate animal doses to human doses. The formula of HED (mg/kg) = dose to be given to the animal (mg/kg) can be used to more accurately determine the human dose equivalent.17 As long as it is not used in large doses, E. longifolia is thought to be harmless. E. longifolia is typically prescribed to be given to men at the standard dose of 200–400 mg daily and should be taken with care, especially in the older people, according to the findings of past toxicity studies. To make daily consumption easier, E. longifolia is currently commercially distributed around the world as tablets following this set dosage.
Conclusions and future prospective
Traditional herbal remedy E. longifolia has a track record of success in vitro in the therapeutics treatment in cancers, which could also present a safer alternative compared to the available chemotherapy. As supported by previous studies, E. longifolia have a high chance to be valuable resources of new drug candidates in the not-too-distant future. However, as the issue with herbal medicines, it is imperative to put an extra attention to the identification, isolation and standardisation of the standardised extract or bioactive compounds. In order to allow a safe clinical usage of E. longifolia, further evaluation on its therapeutic efficacy and safety is necessary besides the available data.
Many plants, including E. longifolia have been used as therapeutic agents for centuries. In fact, though complementary medication may have side effect or indirectly decreases the efficacy of modern medicines, herbal-based medicines and related products continue to rise exponentially with global acceptance. In addition, the legal justification for the usage of herbal drugs remains empirical and hypothetical.48 Hence, medical authorities need to be active in reviewing the regulation protocols and defining the clinical trials, especially on plant-based medications. Global standardisation and more dependable regulatory policies are needed to provide a better platform for these herbal products.
For a fresh advancement in the treatment of cancer, the creation of new phytomedicines and botanical medications requires a continual and systematic approach. In 2008, FDA approved the first herbal ointment, VeregenTM, containing sinecatechins as a partially clean purified entity from the aqueous extract of tea leaves (Camellia sinensis plant). It was used for external (topical) treatment of perianal warts and external genital. In 2013, the FDA approved another herbal drug, Crofelemer, which contains oligomeric proanthocyanidin extracted from Croton lechleri herbal tree for the treatment of diarrhoea in RVD patients. All these examples showed a promising future for herbal medications. Perhaps a global collaboration of pharmaceutical companies may expedite the intervention of E. longifolia based medicines for cancer. Physicians, nurses, and pharmacists generally are inadequate training and knowledge on the mechanism of botanical medicines. Hence, empowering them with sufficient knowledge provides a vital responsibility in monitoring the efficacy and safety of herbal medicines. Collaboration is also needed with the orthodox personnel to create an atmosphere of trust in sharing information and knowledge. In order to improve the health care system and patient compliance, potential negative effects of the plant-based treatment can be avoided.
E. longifolia is one of the most well-known and secure traditional herbal medicines for the treatment of many cancers, and the advancement of natural-based goods is beneficial. More evidence about its clinical treatment, safety, and efficacy is needed in addition to the lab-based research now provided. For the future population’s health, it is crucial to preserve this excellent therapeutic plant.
Acknowledgments
The authors would like to acknowledge University Science Malaysia for their continuous support to prepare this article.
Funding Statement
Funding: None.
References
- 1.Refe Bodeker G, Ong CK. WHO Global Atlas of Traditional, Complementary and Alternative Medicine; World Health Organization: Geneva, Switzerland, 2005; Volume 1. [Google Scholar]
- 2.WHO. Traditional Medicine Strategy 2002–2005; World Health Organization: Geneva, Switzerland, 2002. [Google Scholar]
- 3.Duraz AY, Khan SA. Knowledge, attitudes and awareness of community pharmacists towards the use of herbal medicines in muscat region. Oman Med. J. 2011, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. https://www.cancer.gov . [Google Scholar]
- 5.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017;67:7-30. [DOI] [PubMed] [Google Scholar]
- 6.Keng H. Orders and Families of Malayan Seed Plants. Singapore University Press; Kent Ridge, Singapore: 1978. [Google Scholar]
- 7.Keng H., Keng R.S.L. The Concise Flora of Singapore: GYMNOSPERMS and Dicotyledons. Singapore University Press; Kent Ridge, Singapore: 1990. [Google Scholar]
- 8.Goh S.H., Chuah C., Mok J., Soepadmo E. Malaysian Medicinal Plants for the Treatment of Cardiovascular Diseases. Pelanduk Publications; Petaling Jaya, Malaysia: 1995. [Google Scholar]
- 9.Shaheed Ur Rehman, Kevin Choe, Hye Hyun. Yoo Review on a Traditional Herbal Medicine, Eurycoma longifolia Jack (Tongkat Ali): Its Traditional Uses, Chemistry, Evidence- Based Pharmacology and Toxicology, Molecules. 2016. Mar; 21(3): 331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Christapher Parayil Varghese, Ambrose C., Jin S.C., Lim Y.J., Keisaban T.. (2013). Antioxidant and Anti-inflammatory Activity of Eurycoma Longifolia Jack, A Traditional Medicinal Plant in Malaysia. Int J Pharm Sci Nanotech. 5. 1875-1878. 10.37285/ijpsn.2012.5.4.7. [Google Scholar]
- 11.Kuo P.C., Damu A.G., Lee K.H., Wu T.S. Cytotoxic and antimalarial constituents from the roots of Eurycoma longifolia. Biorg. Med. Chem. 2004, 12, 537–544. [DOI] [PubMed] [Google Scholar]
- 12.Tong KL, Chan KL, AbuBakar S, Low BS, Ma HQ, Wong PF. The in vitro and in vivo anti-cancer activities of a standardized quassinoids composition from Eurycoma longifolia on LNCaP human prostate cancer cells. PLoS One. 2015;10(3):e0121752. Epub 2015/04/01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuo P.C., Damu A.G., Lee K.H., Wu T.S. Cytotoxic and antimalarial constituents from the roots of Eurycoma longifolia. Biorg. Med. Chem. 2004;12:537–544. doi:10.1016/j.bmc.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 14.Darise M., Kohda H., Mizutani K., Tanaka O. Eurycomanone and eurycomanol, quassinoids from the roots of Eurycoma longifolia. Phytochemistry. 1982;21:2091–2093. doi:10.1016/0031-9422(82)83050-1. [Google Scholar]
- 15.Tran T.V.A., Malainer C., Schwaiger S., Atanasov A.G., Heiss E.H., Dirsch V.M., Stuppner H. NF-κB Inhibitors from Eurycoma longifolia. J. Nat. Prod. 2014;77:483–488. doi: 10.1021/np400701k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park S., Nhiem N.X., Van Kiem P., Van Minh C., Tai B.H., Kim N., Yoo H.H., Song J.H., Ko H.J., Kim S.H. Five new quassinoids and cytotoxic constituents from the roots of Eurycoma longifolia. Bioorg. Med. Chem. Lett. 2014;24:3835–3840. doi: 10.1016/j.bmcl.2014.06.058 [DOI] [PubMed] [Google Scholar]
- 17.Food and Drug Administration Guidance for Industry Estimating the Maximum Safe Starting Dose in Initial Critical Trials for Therapeutics in Adult Healthy Volunteers. [(accessed on 3 March 2016)]; Available online: http://www.fda.gov/downloads/Drugs/.../Guidances/UCM078932.pdf [Google Scholar]
- 18.Chan K., Lee S., Sam T., Tan S., Noguchi H., Sankawa U. 13β,18-dihydroeurycomanol, a quassinoid from Eurycoma longifolia. Phytochemistry. 1991;30:3138–3141. doi:10.1016/S0031-9422(00)98272-4. [Google Scholar]
- 19.Miyake K., Tezuka Y., Awale S., Li F., Kadota S. Quassinoids from Eurycoma longifolia. J. Nat. Prod. 2009;72:2135–2140. doi: 10.1021/np900486f. [DOI] [PubMed] [Google Scholar]
- 20.Itokawa H., Qin X.-R., Morita H., Takeya K. C18 and C19 quassinoids from Eurycoma longifolia. J. Nat. Prod. 1993;56:1766–1771. doi: 10.1021/np50100a016. [Google Scholar]
- 21.Ang H.H., Hitotsuyanagi Y., Takeya K. Eurycolactones A–C, novel quassinoids from Eurycoma longifolia. Tetrahedron Lett. 2000;41:6849–6853. doi: 10.1016/S0040-4039(00)01159-X. [DOI] [PubMed] [Google Scholar]
- 22.Low B.S., Teh C.H., Yuen K.H., Chan K.L. Physico-chemical effects of the major quassinoids in a standardized Eurycoma longifolia extract (Fr 2) on the bioavailability and pharmacokinetic properties, and their implications for oral antimalarial activity. Nat. Prod. Commun. 2011;6:337–341. [PubMed] [Google Scholar]
- 23.Gerber B, Scholz C, Reimer T, Briese V, Janni W. Complementary and alternative therapeutic approaches in patients with early breast cancer: a systematic review. Breast Cancer Research and Treatment. 2006;95(3):199–209. [DOI] [PubMed] [Google Scholar]
- 24.Low BS, Choi SB, Abdul Wahab H, Kumar Das P, Chan KL. (2013) Eurycomanone, the major quassinoid in Eurycoma longifolia root extract increases spermatogenesis by inhibiting the activity of phosphodiesterase and aromatase in steroidogenesis. J Ethnopharmacol 149: 201–207. [DOI] [PubMed] [Google Scholar]
- 25.Low BS, Teh CH, Yuen KH, Chan KL. (2011) Physico-chemical effects of the major quassinoids in a standardized Eurycoma longifolia extract (Fr 2) on the bioavailability and pharmacokinetic properties, and their implications for oral antimalarial activity. Nat Prod Commun 6: 337–341. [PubMed] [Google Scholar]
- 26.WHO. WHO Monographs on Selected Medicinal Plants, Vol. 2. 2020. Geneva, Switzerland: World Health Organization. [Google Scholar]
- 27.Rehman S.U., Choe K., Yoo H. H. (2016). Review on a Traditional Herbal Medicine, Eurycoma longifolia Jack (Tongkat Ali): Its Traditional Uses, Chemistry, Evidence- Based Pharmacology and Toxicology. Molecules (Basel, Switzerland), 21(3), 331. 10.3390/molecules21030331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Solomon M., Erasmus N., Henkel R. In vivo effects of Eurycoma longifolia Jack (Tongkat Ali) extract on reproductive functions in the rat. Andrologia. 2014;46:339–348. doi:10.1111/and.12082. [DOI] [PubMed] [Google Scholar]
- 29.Omar Saeed Ali Al-Salahi, Dan Ji, Amin Malik Shah Abdul Majid, Chan Kit-Lam, Wan Zaidah Abdullah, Abdelhamid Zaki, Shah Kamal Khan Jamal Din, Narazah Mohd Yusoff, Aman Shah Abdul Majid. Anti-Tumor Activity of Eurycoma longifolia Root Extracts against K-562 Cell Line: In Vitro and In Vivo Study, Published: January 7, 2014, 10.1371/journal.pone.0083818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Somayeh Fani, Behnam Kamalidehghan, Kong Mun Lo, Najihah Mohd Hashim, Kit May Chow, Fatemeh Ahmadipour. Synthesis structural characterization anticancer activity of a monobenzyltin compound against MCF-7 breast cancer cells, Drug Des Devel Ther 2015 Nov 23;9:6191-201. doi:10.2147/DDDT.S87064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fani S, Dehghan F, Karimian H, Mun Lo K, Ebrahimi Nigjeh S, Swee Keong Y, Soori R, May Chow K, Kamalidehghan B, Mohd Ali H, Mohd Hashim N. Monobenzyltin Complex C1 Induces Apoptosis in MCF-7 Breast Cancer Cells through the Intrinsic Signaling Pathway and through the Targeting of MCF-7-Derived Breast Cancer Stem Cells via the Wnt/β- Catenin Signaling Pathway. PLoS One. 2016. Aug 16;11(8):e0160836. doi: 10.1371/journal.pone.0160836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wong Pooi-Fong, Cheong Wei-Fun, Shu Meng-Hooi, Teh Chin-Hoe, Chan Kit, Abu Bakar, Sazaly. (2011). Eurycomanone suppresses expression of lung cancer cell tumor markers, prohibitin, annexin 1 and endoplasmic reticulum protein 28. Phytomedicine : international journal of phytotherapy and phytopharmacology. 19. 138-44. 10.1016/j.phymed.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 33.Satayavivad J., Noppamas S., Aimon S., Yodhathai T. Toxicological and antimalaria activity of Eurycoma longifolia Jack extracts in mice. Thai J. Phytopharm. 1998;5:14–27. [Google Scholar]
- 34.Singh S, Upadhyay AU, Ajay AK, Bhat MK. p53 regulates ERK activation in carboplatin induced apoptosis in cervical carcinoma: A novel target of p53 in apoptosis. FEBS Lett 581: 289-295, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Yusmazura Zakaria 1, Asmah Rahmat, Azimahtol Hawariah Lope Pihie, Noor Rain Abdullah, Peter J. Houghton Eurycomanone induce apoptosis in HepG2 cells via up-regulation of p53 Cancer Cell Int 2009 Jun 10;9:16 doi: 10.1186/1475-2867-9-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yusmazura Zakaria, Asmah Rahmat, Azimahtol Hawariah Lope Pihie, Noor Rain Abdullah, Peter J Houghton. Eurycomanone induce apoptosis in HepG2 cells via up-regulation of p53, Cancer Cell Int. 2009; 9: 16. Published online 2009 Jun 10. doi: 10.1186/1475-2867-9-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Breuhahn K, Longerich T, Schirmacher P. Dysregulation of growth factor signaling in human hepatocellular carcinoma. Oncogene. 2006;25:3787–3800. [DOI] [PubMed] [Google Scholar]
- 38.Hishikawa K, Oemar BS, Tanner FC, Nakaki T, Luscher TF, Fujii T. Connective tissue growth factor induces apoptosis in human breast cancer cell line MCF-7. J Biol Chem. 1999;274:37461–37466. [DOI] [PubMed] [Google Scholar]
- 39.Cheah S. C., Azimahtol H.L.P. 2004. Eurycomanone exert antiproliferative activity via apoptosis upon MCF-7 cells. Prosiding symposium Biologi Kebangsaan Malaysia ke-7. 73-77. [Google Scholar]
- 40.Chou T, Martin N. CompuSyn for drug combinations: PC software and user’s guide: a computer program for quantitation of synergism and antagonism in drug combinations, and the determination of IC50 and ED50 and LD50 values. ComboSyn, Paramus, NJ. 2005. [Google Scholar]
- 41.Choudhary Y.K., Bommu P., Ming Y.K., Zulkawi N.B. Acute, sub-acute, and subchronic 90-days toxicity of Eurycoma longifolia aqueous extract (Physta) in wistar rats. Int. J. Pharm. Pharm. Sci. 2012;4:232–238. [Google Scholar]
- 42.Kajahmohideen Nur Haseena, Siti NurSyafiqah Razi, Faisal Ghasak Ghazi, Ashour, Abdelkader Elbadawy Abbas. (2020) Cytotoxic activity of eurycoma longifolia jack against nasopharyngeal carcinoma cell lines. In: 9th Dental Students' Scientific Conference. 2020, 24th February 2020, Kuantan, Pahang [Google Scholar]
- 43.Nurkhasanah Mahfudh, Azimahtol Hawariah Lope Pihie. Eurycomanone Induces Apoptosis through the UpRegulation of p53 in Human Cervical Carcinoma Cells Journal of Cancer Molecules 4(4): 109-115, 2008. [Google Scholar]
- 44.Thu HE, Hussain Z, Mohamed IN, Shuid AN. Eurycoma longifolia, A Potential Phytomedicine for the Treatment of Cancer: Evidence of p53-mediated Apoptosis in Cancerous Cells. Curr Drug Targets. 2018;19(10):1109-1126. doi:10.2174/1389450118666170718151913 [DOI] [PubMed] [Google Scholar]
- 45.Pooi-Fong Wong 1, Wei-Fun Cheong, Meng-Hooi Shu, Chin- Hoe Teh, Kit-Lam Chan, Sazaly AbuBakar. Eurycomanone suppresses expression of lung cancer cell tumor markers prohibitin annexin 1 and endoplasmic reticulum protein 28, Phytomedicine, 2012 Jan 15;19(2):138-44. doi:10.1016/j.phymed.2011.07.001 [DOI] [PubMed] [Google Scholar]
- 46.Bhasin S., Cunningham G.R., Hayes F.J., Matsumoto A.M., Snyder P.J., Swerdloff R.S., Montori V.M. Testosterone therapy in men with androgen deficiency syndromes: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2010;95:2536–2559. doi: 10.1210/jc.2009-2354. [DOI] [PubMed] [Google Scholar]
- 47.Li C.H., Liao J.W., Liao P.L., Huang W.K., Tse L.S., Lin C.H., Kang J.J., Cheng Y.W. Evaluation of Acute 13-Week Subchronic Toxicity and Genotoxicity of the Powdered Root of Tongkat Ali (Eurycoma longifolia Jack) Evid. Based Complement. Altern. Med. 2013;2013 doi: 10.1155/2013/102987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Park S., Nhiem N.X., Van Kiem P., Van Minh C., Tai B.H., Kim N., Yoo H.H., Song J.H., Ko H.J., Kim S.H. Five new quassinoids and cytotoxic constituents from the roots of Eurycoma longifolia. Bioorg. Med. Chem. Lett. 2014, 24, 3835–3840 [DOI] [PubMed] [Google Scholar]


