Highlights
-
•
Fast, simple and inexpensive procedure for detecting potential wine adulterations.
-
•
Aged wine authentication on the basis of digital image information and chemometrics.
-
•
Quantitative prediction of adulteration levels of aged wines using PLS regression.
Keywords: Aged-wine, Authentication, Digital image, RGB-colorgram, Multivariate analysis
Abstract
A method has been developed to authenticate aged high-quality wines and to quantify their potential adulterations through multivariate analysis and regression techniques applied to the obtained RGB digital images. Wines of pure Gran Reserva, Crianza, and Joven Rioja as well as synthetic adulterated Gran Reserva samples were studied. Digital images were obtained by a single and inexpensive lab-made device. Each sample was characterized by means of the 256 channels intensities from the RGB-colorgram. Multivariate image analysis revealed differences among the wine classes, and between genuine-aged and adulterated samples. Partial least squares regression was used to develop a model for estimating the adulteration degree of Gran Reserva wines. The model achieved good prediction (RMSEP = 1.6), appropriate precision (RSD = 2.5%) and suitable LOD (2.3%) to quantify cost-effective adulterations. The present method, due to simplicity and low cost, could provide an appropriate alternative to the traditional chemical authentication methods.
1. Introduction
The issue of food product and beverage authenticity constitutes an active research field in food science (Schieber, 2018). Food authentication is the practice that verifies if a food is in compliance with its label description. When the label of a product include a declaration of specific quality, this results in high-added value merchandise, and therefore it is often target of adulteration, falsification and mislabeling (Danezis, Tsagkaris, Camin, Brusic, & Georgiou, 2016). In the case of high-quality wines with Protected Designation of Origin (PDO) potential adulterations include dilution, addition of alcohol or prohibited substances, forbidden aging methods, unauthorized grape varieties, mislabeling and replacement or mixing with lower quality wines.
Rioja is a Spanish wine-region with PDO well-known for its red wines from Tempranillo variety. In this region, high quality wines, classed as Gran Reserva (GR), are selected from exceptional vintages and they require an aging procedure of at least two years in oak and three in bottle. GR are expensive wines and therefore they can be adulterated by mixing them with other cheaper and younger wines from the same variety, such as Crianza (C): one year in oak and a few months in bottle wine, or Joven (Y) wines in their first or second year. Therefore, fast and accurate procedures for ensuring the product genuineness and for detecting adulterations are required by producers, consumers and authorities to protect the brand image, to prevent overpayment, and to preserve the product quality.
Visual characteristics are important parameters for wine quality. In fact, the development of objective procedures to measure visual properties and to relate them with composition or quality is an interesting approach in food science (Foca, Masino, Antonelli, & Ulrici, 2011). Recently, the use of digital images providing appropriate color data for this task increased dramatically due to the availability of inexpensive instruments as digital cameras, phones, or scanners. Different color spaces (RGB: red, green, blue; HSV: hue, saturation, value; or CMYK: cyan, magenta, yellow, black) were employed for the construction of a data matrix with color characteristics from a set of images. The numerical information from color spaces was processed in combination with chemometric tools with two different approaches: Multivariate Image Analysis (MIA) and Multivariate Image Regression (MIR). MIA (Geladi, Isaksson, Lindqvist, Wold, & Svenson, 1989) involves the application of principal component analysis (PCA) to digital images to achieve an unsupervised exploration of image characteristics in the score-space by principal components. On the other hand, MIR is defined as the use of different multivariate regression procedures based on features from digital images to predict any product property (Duchesne, Liu, & MacGregor, 2012).
In the last decade different research papers have been published on these two strategies in which the information from digital images has been processed for the determination of sensory properties of food commodities as well as diverse components and/or undesired contaminants: colorants and color-related compounds (Botelho et al., 2014, Domínguez and Centurión, 2015, Foca et al., 2011, Vidal et al., 2018), antibiotics (Urapen and Masawat, 2015a, Urapen and Masawat, 2015b) and heavy metals (Lutfi-Firdaus et al., 2014, Nguyen et al., 2018). Other different applications are reviewed in the literature by Duchesne et al. (2012) and Wu and Sun (2013). As illustrated by the selected examples summarized above, composition and sensory properties of foods and beverages have been predicted using digital image-based methods. However, only few applications in food authentication are published to date, among them: the detection and quantification of bovine milk adulteration (Macedo dos Santos and Rodrigues Pereira-Filho, 2013, Macedo dos Santos et al., 2012), and the authentication of Argentinean honeys from different geographical origins (Domínguez, Gonçalves Dias Diniz, Di Nezio, Uglino de Araujo, & Centurion, 2014).
In this work, digital images were employed to detect and quantify adulteration of GR Rioja wines. The MIA-MIR systems developed, based on RGB-information, were successfully used to detect adulteration of GR wines and to quantify the percentage of younger wines present in adulterated samples.
2. Material and methods
2.1. Wine samples
All the wine samples used belong to Rioja PDO, and they have been elaborated employing Tempranillo grapes, the most common variety in Rioja (Ramos & Martínez de Toda, 2019). The sample origin is guaranteed by the Directive Council of this PDO through the corresponding back label. Samples with three different aging levels and quality (Gran Reserva, Crianza, and Joven) were arranged in three different sample sets, as detailed in Table 1. PURE-set and ADULTER-set were used for MIA analysis, while PLSR-set was employed in MIR analysis.
Table 1.
Description of the sample sets used in this work, and analytical figures of merit of the developed Partial Least Squares Regression model.
| Sample set | Total samples | Class of samples | Used for | Dimension of the data matrix obtained and notes |
|---|---|---|---|---|
| PURE-set | 25 |
|
Multivariate Image Analysis | 25 × 256 (25 samples and 256 RGB variables) |
| ADULTER-set | 78 |
|
Multivariate Image Analysis | 78 × 256 (78 samples and 256 RGB variables) |
| PLSR-set | 52 |
|
Multivariate Image Regression | 52 × 257 (52 samples and 256 RGB variables plus a Y-variable of % adulteration) For validation, this set was divided into two subsets: calibration (40) and test (12). See details in the text. |
| Model | Latent Factors | Range (%) | RMSE (Root mean square error) |
RSD* (%) | Detection limit (%) | ||
|---|---|---|---|---|---|---|---|
| RMSE of Cross Validation | RMSE of Calibration | RMSE of Prediction | |||||
| PLSR | 6 | 0–50 | 1.7 | 0.99 | 1.6 | 2.5 | 2.3 |
Relative standard deviation.
PURE-set was composed of 25 genuine Rioja wines of three different classes: Six GR wines were 2009 harvest replicates from different bottles of the same commercial brand; six C wines were of two different brands, harvested in 2014 and 2015; and the thirteen Y wines are samples belonging to 5 different brands from 2016 and 2017 years. This sample set was employed in order to explore if the RGB-data contained useful information to differentiate between classes.
The second ADULTER-set included 72 synthetic adulterated GR samples plus the 6 genuine GR samples. Adulterated GR samples were prepared by mixing genuine GR wine samples with each different Crianza (3) and Joven (5) wines at three different adulteration levels (10, 20 and 30%). In all cases, adulterated samples were prepared and measured in triplicate. This set was used to evaluate if the adulterated wines formed a separate group from authentic GR samples in the multidimensional space of RGB-data.
Finally, the MIR approach was developed on the basis of color information of the samples contained in PLSR-set. This set is composed of 52 GR adulterated samples that were prepared by mixing a genuine GR wine sample (2009 harvest) with different quantities of a Rioja young wines (from 2017 harvest). The adulteration levels were in the range from 0 to 50% (in 2% intervals), and the samples were prepared and measured in duplicate. In this case, the complete set was used for studying if the adulteration level of the samples can be predicted by a PLS regression model on the basis of RGB information.
2.2. Apparatus and software
Digital images were obtained in a lab-made system (see Fig. 1) under controlled light using a white LED. The system included a digital camera (Canon-50D) operated by a computer using the software supplied by the manufacturer (EOS Utility 2.14.10) and equipped with a Sigma 105 mm objective, F:2.8-Macro. Samples were placed in a prismatic PTFE-cuvette (75 × 60 × 5 mm) in a holder at fixed distance (15 cm) from the camera focal plane. Digital images were processed to obtain RGB-data using the image scientific software ImageJ, ver.1.52a, developed by National Health Institute, USA (available at http://imagej.nih.gov/ij). Chemometric procedures were performed using Statgraphics Centurion XVI ver.16.1.15 (Statistical Graphics, Rockville, MD, USA).
Fig. 1.
Schematic representation of the lab-made system used for digital image measurement of beverage samples. 1: Digital reflex camera; 2: Fixed holder and cuvette; 3: Closed box for light control; 4: LED lamp; and 5: Control computer.
2.3. Digital imaging technique
Digital images were obtained by placing aliquots of the pure and adulterated wine samples in the cuvette of the lab-made system described above. In all cases fixed manual focus (focusing on the central point of the cuvette covering 90% of the total surface), fixed custom white balance, F:5.6 and exposure time of 1 s were used. Images were obtained by remote operation from the computer (avoiding undesired movements of the camera) and they were directly stored as uncompressed jpeg files. Jpeg image format was selected over other formats after preliminary assays in which it was demonstrated that the RGB-histograms obtained contained comparable information to those in large raw files. Thus, jpeg files retained the residing color information and allowed ease of handling due to the smaller size. Colorgrams composed of the intensity of each of the 256 RGB-channels were obtained from digital images using ImageJ. The specific design of the lab-made system provided digital pictures completely homogeneous over the whole surface of the image. This approach avoided manual collection by the operator of sets of selected pixels with the appropriate color from the image (as it occurs for other image-capture devices with wider focal range), and therefore RGB-colorgrams were obtained from the complete images without any manipulation or partial selection. Examples of RGB-colorgrams for several GR, C and Y wines are presented in Fig. S1.
2.4. RGB-data and chemometric procedures
For each set of samples, different mx256 matrices were prepared (See Table 1) from the RGB-colorgram data of each digital image. In all cases, rows (m) corresponded to wine samples, while columns (features) were the numerical data for the 256 RGB-channels. Data matrices were subjected to different multivariate chemometric procedures to explore the data and to build diverse mathematical models. In the first step of multivariate image analysis, principal component analysis (PCA) was applied to the data matrices obtained from PURE-set and ADULTER-set. PCA is a dimension-reduction technique that transforms the original matrix Xmx256 into a product of two matrices, one containing information about the variables (score-matrix Smx256) and the other about variables (loadings-matrix L256x256). If the number of principal components selected is small compared to number of original variables, PCA provides a considerable dimension reduction and simplification of the original matrix. In addition, projection of samples in the space of the few selected principal components (score-plot) allows a visual evaluation of the location of the samples in the multidimensional space, revealing latent structures and relations between samples (Joliffe, 2002). In the second stage of multivariate image regression, partial least squares regression (PLSR) was employed as regression technique to quantify adulterations. PLSR is a method based on the derivation of the complex relationship between a set of predictor variables (X variables) and one or more dependent variables (Y responses). PLSR extracts a reduced number of latent factors (LF) for prediction (determined by cross-validation) associated with directions in the factor space related to high variation in the Y-responses. LFs are biased to obtain the best prediction (Geladi and Kowalski, 1986, Tobias, 1995). In the case at hand, X-variables were the RGB-data and Y-responses were the adulteration levels. For this task, the data matrix obtained from the PLSR-set was used.
3. Results and discussion
3.1. Multivariate image analysis
With the aim of evaluating whether RGB-data contained useful information to achieve color differentiation between GR, C and Y wines, the 25x256 data matrix obtained from PURE-set was subjected to PCA. RGB-variables were autoscaled in order to avoid the effect of the different sizes of the features. Inactive channels were removed from the analysis. The two first principal components extracted represented 83.5% of the data variance, which was considered acceptable to represent the data matrix. The score-plot obtained in this reduced space of two components is presented in Fig. 2. It can be seen that color evolution from Young, through Crianza, to Gran Reserva wines is observed in this score-space. The color of wines is influenced by the content of phenolic compounds, which is related to aging time. In young wines the color is hardly conditioned by the content of free anthocyanins. In general, when a wine is subjected to an aging process, the color density diminishes due to oxidation reactions, and changes in the structure of anthocyanin-tannin polymers (Basalekou et al., 2017). These color changes can be detected, in certain cases, by an expert eye-trained taster, but in general they are very difficult to observe. However, this change is clearly evidenced by RGB-colorgrams. In this case, this evolution is demonstrated by a curved line from negative-positive values of PCOMP-1 and negative-positive-negative values for PCOMP-2 in Fig. 2. Consequently, it is clear that differences in RGB-color between the three wine classes could be used in differentiation studies.
Fig. 2.
2D-score plot of PURE-set samples obtained from PCA.
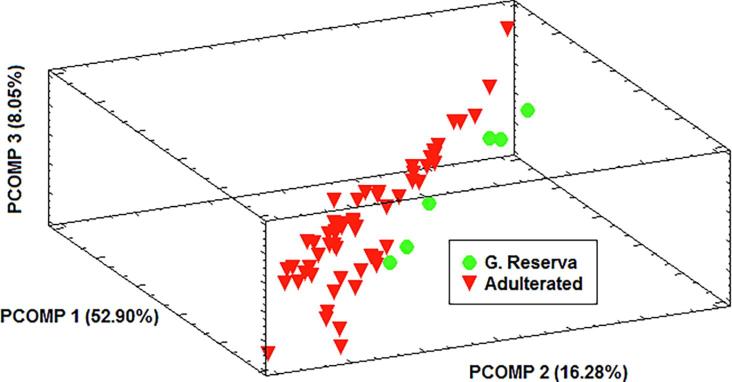
The second step in MIA involved an assay to confirm that these class-color differences are potentially useful to detect adulterations. In other words, whether the color change produced is sufficient to confirm that adulteration has taken place when GR wine is adulterated with different quantities of C and Y wines. With this aim in mind, a new set of wines ADULTER-set was utilized. Samples of this set (6 GR pure Rioja wines and 72 adulterated samples) were measured according to the procedure previously described and the RGB-colorgrams were obtained. The resulting 78x256 matrix was subjected to the data autoscaling pretreatment outlined above. The 3D-score plot (77.23% of the total variance) obtained by PCA is presented in Fig. 3. It can be seen that there is a subtle but significant difference in the locations of adulterated and pure GR samples in this score-space. This indicates that, on the basis of RGB-information, the extremely small change in color of pure GR samples when they were adulterated with non-GR samples could be appropriate to detect adulterations.
Fig. 3.
3-D score plot of ADULTER-set samples obtained from PCA.
3.2. Multivariate image regression
Once it had been demonstrated that the color of wines constitutes a useful tool to differentiate between pure and adulterated GR wines, the next step was to apply MIR in order to develop a system capable of predicting the adulteration level. Nevertheless, prior to multivariate approach, a univariate study was carried out to verify if any RGB-channel could be appropriately related with the adulteration level. It was confirmed that none of the RGB-channels by itself can be used for this objective. Consequently, the multivariate approach was explored. Therefore, the 52x257 data matrix from the PLSR-set was subjected to PLSR in order to develop a mathematical model to predict the level of adulteration on the basis of RGB-colorgrams. The predictive capability for the developed multivariate regression models must be evaluated on samples other than those used for the model construction by using an external validation procedure (Berrueta, Alonso-Salces, & Heberger, 2007). For this task, data sets are commonly divided into two subsets: the training or calibration set, a collection of samples employed to build up the model; and the test or prediction set, an independent series of samples (with well-known output and not used in training step) employed in order to assess if the predictions made by the model were correct. Root mean square error was applied in cross-validation for the selection of the optimum number of LFs in PLSR (RMSECV) as well as for evaluating the performance of the selected PLSR model, both in calibration (RMSEC) on the training set and in prediction (RMSEP) on the test set (see Supplementary material for detailed description). In the case at hand, 40 samples were used as training set while test set was composed of 12 independent samples. Selection of the number of optimal latent variables for the PLSR model was carried out by cross-validation on the calibration set. Leave-one-out cross-validation was employed and models were developed with 39 samples and the remaining one was predicted. This procedure was repeated 40 times in order to ensure that all samples were represented in both subsets. The results obtained for RMSECV of the different PLSR models with diverse number of LFs (up to 15) are presented in Fig. 4. According to these results, a model rank of six LFs was selected as appropriate because the RMSECV-error achieved the minimum and the retained variances in X and Y were higher than 95%. Selection of a high number of LFs could produce slightly better results but this would give an undesirable risk of data over-fitting (Faber & Rajk, 2007). With this LF arrangement, the PLSR model was developed on the basis of 40 calibration samples (in an adulteration range from 0 to 50%) and 12 samples as an external validation set (in the range 2–48%) were used to predict the percentage of adulteration. The calibration line obtained (Fig. 5a) showed confidence intervals for slopes and the intercepts of the regressions including 1 and 0, respectively. Thus, no significant differences between the percentage of adulteration predicted by PLSR and the true value were demonstrated. In addition, the calibration line showed a good correlation coefficient (R = 0.998) and the error as RMSEC was 0.99. This is an interesting result because it means that the colorgrams obtained include valuable information to develop a multivariate model to study the adulteration of GR wines from a single image. The prediction ability was tested by applying the PLSR-model to the external test set by predicting the % of adulteration for 12 adulterated GR-samples (2, 8, 18, 28, 38 and 48% of adulteration). The result obtained on this prediction set (Fig. 5b) for the regression line of the PLSR-predicted against the true level of adulteration is a straight line (R = 0.995) with slope 1 and an intercept 0. The error of prediction as RMSEP gave a satisfactory value of 1.6. The PLS-based regression was shown to produce a linear response for adulteration levels up to 50%. The limit of detection of the prediction model (see Supplementary material for description) was 2.3%, which is sufficient to detect adulterations in an economically viable way. The relative standard deviation (RSD) for triplicate measurements at 25 different concentration levels (from 2 to 50%) ranged from 0.22 to 3.8%, with a mean RSD of 2.5%. A summary of the analytical figures of merit for the present PLSR procedure is presented in Table 1. It has been clearly demonstrated that RGB images in combination with multivariate regression procedures is applicable for quantifying adulterations in GR wines.
Fig. 4.
Percentage of X- and Y-retained variance as well as RMSECV for PLSR models based on different numbers of latent factors.
Fig. 5.
True percentage of adulteration vs PLSR-predicted percentages of adulteration for calibration (a) and validation (b) sets. RMSEC: Root mean square error of calibration; RMSEP: Root mean square error of prediction; R: correlation coefficient.
4. Conclusion
MIA and MIR approaches based on RGB-information on the color of wines can be exploited by applying chemometric multivariate procedures in order to detect and quantify adulterations of high-quality wines. MIA-PCA revealed differences in digital images obtained from pure and adulterated samples, and the MIR-PLSR model developed produced suitable results in terms of prediction for the percentage of adulteration of GR wine (up to 50%). In addition, the use of the complete digital image, avoiding any pre-treatment or partial selection by the operator, is an advantage of the procedure. When the appropriate model is built for the corresponding GR wine, authentication of genuine samples of this Rioja class could be carried out quickly by non-experts personnel in the field. Therefore, this tool due to its simplicity and low-cost could be applied in control laboratories as a first and fast step for the detection of wine fraud. To our knowledge, this is the first case in which RGB-images have been used to evaluate wine authentication. However, this is a preliminary work and deeper studies will be necessary on a more extent set of GR samples of different aging times in order to test the potential generalization of the idea here developed. Finally, taking into account the increasing capability of computer calculations and storage, and the possibility of acquiring high-resolution images with low noise levels using inexpensive devices, further applications based on this approach should be expected in the future for different food products.
Declaration of Competing Interest
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2019.100046.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Basalekou M., Pappas C., Kotseridis Y., Tarantilis P.A., Kontaxakis E., Kallithraka S. Red wine age estimation by the alteration of its color parameters: Fourier transform infrared spectroscopy as a tool to monitor wine maturation time. Journal of Analytical Methods in Chemistry. 2017;5767613 doi: 10.1155/2017/5767613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrueta L.A., Alonso-Salces R.M., Heberger K. Supervised pattern recognition in food analysis. Journal of Chromatography A. 2007;1158:196–214. doi: 10.1016/j.chroma.2007.05.024. [DOI] [PubMed] [Google Scholar]
- Botelho B.G., de Asis L.P., Sena B.M. Development and analytical validation of a simple multivariate calibration method using digital scanner images for sunset yellow determination in soft beverages. Food Chemistry. 2014;150:175–180. doi: 10.1016/j.foodchem.2014.03.048. [DOI] [PubMed] [Google Scholar]
- Danezis G.P., Tsagkaris A.S., Camin F., Brusic V., Georgiou C.A. Food authentication: Techniques, trends and emerging approaches. Trends in Analytical Chemistry. 2016;85:123–132. [Google Scholar]
- Domínguez M.A., Centurión M.E. Application of digital images to determine color in honey samples from Argentina. Microchemical Journal. 2015;118:110–114. [Google Scholar]
- Domínguez M.A., Gonçalves Dias Diniz P.H., Di Nezio M.S., Uglino de Araujo M.C., Centurion M.E. Geographical classification of Argentinean honeys using a digital image-based flow-batch system. Microchemical Journal. 2014;112:104–108. [Google Scholar]
- Duchesne C., Liu J.J., MacGregor J.F. Multivariate image analysis in the process industries: A review. Chemometrics and Intelligent Laboratory Systems. 2012;117:116–128. [Google Scholar]
- Faber N.M., Rajk R. How to avoid over-fitting in multivariate calibration. The conventional validation approach and an alternative. Analytica Chimica Acta. 2007;595:98–106. doi: 10.1016/j.aca.2007.05.030. [DOI] [PubMed] [Google Scholar]
- Foca G., Masino F., Antonelli A., Ulrici A. Prediction of compositional and sensory characteristics using RGB digital images and multivariate calibration techniques. Analytica Chimica Acta. 2011;706:238–245. doi: 10.1016/j.aca.2011.08.046. [DOI] [PubMed] [Google Scholar]
- Geladi P., Isaksson H., Lindqvist L., Wold S., Svenson K. Principal component analysis of multivariate images. Chemometrics and Intelligent Laboratory Systems. 1989;5:209–220. [Google Scholar]
- Geladi P., Kowalski B.R. Partial least-squares regression: A tutorial. Analytica Chimica Acta. 1986;185:1–17. [Google Scholar]
- Joliffe I.T. 2nd ed. Springer; New York: 2002. Principal component analysis. [Google Scholar]
- Lutfi-Firdaus M., Alwi W., Trinoveldi F., Rahayu I., Rahmidar L., Warsito K. Determination of Chromium and Iron using digital image-based colorimetry. Procedia Environmental Sciences. 2014;20:298–304. [Google Scholar]
- Macedo dos Santos P., Rodrigues Pereira-Filho E. Digital image analysis – An alternative tool for monitoring milk authenticity. Analytical Methods. 2013;5:3669–3674. [Google Scholar]
- Macedo dos Santos P., Wentzell P.D., Rodrigues Pereira-Filho E. Scanner digital images combined with color parameters: A case study to detect adulterations in liquid cow’s milk. Food Analytical Methods. 2012;5:89–95. [Google Scholar]
- Nguyen H., Sung Y., O’Shaughnessy K., Shan X., Shih W.C. Smartphone nanocolorimetry for on-demand lead detection and quantification in drinking water. Analytical Chemistry. 2018;90:11517–11522. doi: 10.1021/acs.analchem.8b02808. [DOI] [PubMed] [Google Scholar]
- Ramos M.C., Martínez de Toda F. Variability of Tempranillo grape composition in the Rioja DOC (Spain) related to soil and climatic characteristics. Journal of the Science of Food and Agriculture. 2019;99:1153–1165. doi: 10.1002/jsfa.9283. [DOI] [PubMed] [Google Scholar]
- Schieber A. Introduction to food authentication. In: Sun Da-Wen., editor. Modern techniques for food authentication. Elsevier; Amsterdam, Holland: 2018. pp. 1–21. [Google Scholar]
- Tobias R. Proceedings of the twentieth annual SAS users group international conference. SAS Institute Inc.; Orlando, FL: 1995. An introduction to partial least squares regression; pp. 1250–1257. [Google Scholar]
- Urapen R., Masawat P. Novel method for the determination of tetraciclyne in bovine milk based on digital-image-based colorimetry. International Dairy Journal. 2015;44:1–5. [Google Scholar]
- Urapen R., Masawat P. An iPhone-based digital image colorimeter for detecting tetracycline in milk. Food Chemistry. 2015;184:23–29. doi: 10.1016/j.foodchem.2015.03.089. [DOI] [PubMed] [Google Scholar]
- Vidal M., García-Arrona R., Bordagaray A., Ostra M., Albizu G. Simultaneous determination of color additives tartrazine and allura red in food products by digital image analysis. Talanta. 2018;184:58–64. doi: 10.1016/j.talanta.2018.02.111. [DOI] [PubMed] [Google Scholar]
- Wu D., Sun D.W. Colour measurements by computer vision for food quality control – A review. Trends in Food Science & Technology. 2013;29:5–20. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.