Abstract
Background
Individuals engage in a range of behaviors to maintain close relationships. One behavior is self-silencing or inhibiting self-expression to avoid relationship conflict or loss. Self-silencing is related to poor mental health and self-reported physical health in women but has not been examined in relation to cardiovascular health, particularly using direct measures of the vasculature.
Purpose
To test associations between self-silencing and carotid atherosclerosis in midlife women; secondary analyses examined moderation by race/ethnicity.
Methods
Women (N = 290, ages 40–60) reported on self-silencing in intimate relationships and underwent physical measurements, blood draw, and ultrasound assessment of carotid intima–media thickness (IMT) and plaque. Associations between self-silencing and mean IMT and plaque index (0, 1, ≥2) were tested in linear regression and multinomial logistic regression models, respectively, followed by interaction terms between self-silencing and race, adjusted for demographic factors, CVD risk factors, partner status, depression, physical activity, and diet.
Results
Forty-seven percent of women demonstrated carotid plaque. Greater self-silencing was related to increased odds of plaque index ≥2 (e.g., for each additional point, odds ratio [95% confidence interval] = 1.16 [1.03–1.31], p = .012), relative to no plaque). Moderation analyses indicated that self-silencing was related to odds of plaque index ≥2 in non-white women (1.15 [1.05–1.26], p = .004), but there was no significant relationship in white women (1.01 [0.97–1.06], p = .550). No associations emerged for IMT.
Conclusions
Among midlife women, self-silencing was associated with carotid plaque, independent of CVD risk factors, depression, and health behaviors. Emotional expression in relationships may be important for women’s cardiovascular health.
Keywords: Atherosclerosis, Cardiovascular diseases, Carotid plaque, Self-silencing, Emotional expression, Women
Among midlife women, self-silencing of emotional expression was associated with carotid plaque, independent of cardiovascular disease risk factors, depression, and health behaviors.
Introduction
A wealth of research indicates that social relationships are important for cardiovascular health [1, 2]. Close intimate relationships (e.g., marriage, romantic relationships) appear to be particularly impactful [3, 4]. Notably, cardiovascular disease (CVD) is the leading cause of morbidity and mortality among women [5]. Gendered social experiences, including gender role expectations for women to prioritize the needs of others and to create and maintain close relationships [6], may be related to CVD risk in women [7, 8].
How women express themselves and assert their needs, particularly within intimate relationships, may be related to health. In some relationships, such as those with a greater power differential, women may learn to self-silence, or inhibit one’s feelings, thoughts, or actions to avoid conflict or loss of relationship [6, 9]. Greater self-silencing has been related to poor mental health and self-reported physical health in women, including symptoms of depression [9–12], disordered eating [13, 14], and irritable bowel syndrome [15] (see [16] for a comprehensive review).
However, it is not known whether self-silencing is associated with women’s cardiovascular health, particularly using direct measures of the vasculature. Vascular imaging allows for measurement of underlying vascular health among individuals without clinical CVD. Use of these measures is important for understanding midlife women’s cardiovascular health given the low rate of clinical CVD among women at midlife [17]. Well-validated indices of subclinical CVD include ultrasound-derived carotid intima–media thickness (IMT), an index of the thickness of the intimal and medial layers of the carotid artery [18], and presence and extent of carotid plaque, a measure of focal atherosclerotic lesions [18]. Carotid IMT and plaque are known to predict later CVD events, including among relatively low-risk samples, such as midlife women [18–20].
In a community sample of nonsmoking midlife women free of clinical CVD, we tested whether self-silencing in one’s current or last intimate relationship was related to higher carotid IMT and plaque, controlling for a range of potentially confounding demographic factors, standard CVD risk factors, partner status, depressive symptoms, physical activity, and dietary intake. Additional analyses examined whether associations differed by race/ethnicity.
Methods
Sample
This study was conducted among participants of a study initially designed to examine the relationship between menopausal symptoms and cardiovascular health [21]. The study was conducted between January 2012 and May 2015 and included 304 nonsmoking women aged 40–60. Women were recruited from the greater Pittsburgh area via advertisements, mailings, fliers, and message boards.
Exclusion criteria included hysterectomy and/or bilateral oophorectomy; a reported history of heart disease, stroke, arrhythmia, ovarian/gynecological cancer, pheochromocytoma, pancreatic tumor, kidney failure, seizures, Parkinson disease, Raynaud phenomenon; current pregnancy; or having used the following medications in the past three months: estrogen or progesterone, selective estrogen receptor modulators, selective serotonin reuptake inhibitors, serotonin–norepinephrine reuptake inhibitors, gabapentin, insulin, β-blockers, calcium channel blockers, α-2 adrenergic agonists, and other antiarrhythmic agents. Women who were undergoing chemotherapy, hemodialysis, or peritoneal dialysis were also excluded [21].
Of the 304 women, 14 women were excluded due to missing self-silencing data (n = 7), carotid data (equipment failure or poor image; plaque: n = 2; IMT: n = 5) or select blood marker data (homeostatic model assessment [HOMA]: n = 3, low-density lipoprotein cholesterol [LDL-C]: n = 2). Excluded women had higher triglycerides, lower self-silencing scores, and were younger (ps < .05) than included women. A total of 290 and 287 women were included in the final models for carotid plaque and IMT, respectively.
Design and Procedures
Participants underwent telephone screening for study eligibility and completed physical measurements, a blood draw, a carotid artery ultrasound scan, and a computer-administered psychosocial questionnaire battery (including self-silencing). Procedures were approved by the University of Pittsburgh Institutional Review Board. Participants provided written informed consent.
Measures
Self-silencing
Self-silencing was assessed via a modified 12-item version of the Silencing the Self Scale (STSS) which measures self-expression and assertion in one’s current or previous intimate relationship (if not currently in an intimate relationship) on a scale from 1 (strongly disagree) to 5 (strongly agree) [6, 9]. The STSS yields a total scale score as well as four subscale scores, each measured via 3 items: Self-Silencing Subscale = inhibiting self-expression and action to avoid conflict and loss of relationship (e.g., “I rarely express my anger at those close to me”); Externalized Self-Perception Subscale = seeing and judging the self by external standards (e.g., “I find it hard to know what I think and feel because I spend a lot of time thinking about how other people are feeling”); Care as Self-Sacrifice Subscale = securing attachments by placing the needs of others before the self (e.g., “Caring means putting the other person’s needs in front of my own”); and Divided Self Subscale = presenting an outward self that differs from inner experience (e.g., “For my partner to love me, I cannot reveal certain things about myself”). Items were summed to create a STSS total score (possible range: 12–60) and four subscale scores (possible range: 3–15); higher scores = greater self-silencing. STSS total score was reliable in the present investigation (Cronbach’s α = .71).
Carotid plaque
Trained and certified sonographers at the University of Pittsburgh Ultrasound Research Laboratory obtained bilateral carotid images via B-mode ultrasound using a Sonoline Antares (Siemens, Malvern, PA, USA) high-resolution duplex scanner equipped with a VF10-5 transducer according to a standardized protocol [22]. Digitized images were obtained from eight locations (four locations each from the left and right carotid arteries): near and far walls of the distal common carotid artery, far walls of the carotid bulb, and internal carotid artery. Images were read using semiautomated reading software [23]. Values were obtained by electronically tracing the lumen–intima interface and the media–adventitia interface across a 1-cm segment for each of the eight segments. Average values were recorded for each of the eight locations; the mean of the average readings across these locations comprised mean IMT. Reproducibility of IMT measures was excellent in this study (intraclass correlation coefficient between sonographers = 0.87–0.94, between readers = 0.94–0.99).
Sonographers evaluated the presence and extent of carotid plaque in each of five segments of the left and right carotid artery (distal and proximal common carotid artery, carotid bulb, and proximal internal and external carotid arteries) [24]. Consistent with the Mannheim Consensus Statement, plaque was defined as a distinct focal area protruding into the vessel lumen that was at least 50% thicker than the adjacent IMT and summarized as the presence or absence of any plaque [25]. Additionally, for each segment, the degree of plaque was graded as follows: grade 0 = no observable plaque; grade 1 = 1 small plaque (<30% of the vessel diameter); grade 2 = 1 medium plaque (30%–50% of the vessel diameter) or multiple small plaques; grade 3 = 1 large plaque (>50% of the vessel diameter) or multiple plaques with at least one medium plaque. The grades from all segments of the combined left and right carotid artery were summed to create the plaque index [26], which was categorized as 0, 1, ≥2 for analysis. Between-sonographer agreement for carotid plaque assessment was good to excellent in this study (κ statistic, κ = 0.78).
Covariates
Height and weight were measured via a fixed stadiometer and balance beam scale; body mass index (BMI) was calculated (kg/m2). Seated blood pressure was measured via a Dinamap device after 10-min rest. Demographics and medical history were assessed by standard instruments, including use of medications for blood pressure-lowering, lipid-lowering, or diabetes mellitus. Menopause status was obtained from reported menstrual bleeding patterns [27]. Depressive symptoms were assessed by the Center for Epidemiologic Studies Depression (CESD) scale and were categorized according to the clinical cut point (CESD ≥16) [28]. Physical activity over the last 7 days (metabolic equivalent of task [MET] minutes/week) was assessed via the International Physical Activity Questionnaire (IPAQ) leisure-time physical activity subscale [29] and usual dietary intake over the past year (total kilocalories) was measured via the Block Brief 2000 Food Frequency Questionnaire [30]. Race/ethnicity was categorized as white versus non-white; the vast majority (80%) of non-white women identified as African American. Women reported partner status (categorized in this analysis as: currently married and living together or living with someone in a marital-like relationship; widowed, separated, or divorced/formerly lived with someone in a marital-like relationship; never married/never lived with someone in a marital-like relationship). Phlebotomy was performed after a 12-hr fast. Triglycerides and total cholesterol were measured enzymatically and LDL-C calculated [31]. HOMA, reflecting insulin resistance, was calculated [32].
Data Analysis
Due to skewness, values for triglycerides, HOMA, dietary intake, and physical activity (after adding 1) were natural log-transformed prior to analysis. Transforming triglycerides and HOMA is the standard procedure in epidemiological investigations because of the skewed nature of the data [33, 34]. Unadjusted correlations were conducted to examine associations between STSS total score and study variables. Analysis of variance, t-tests, and chi-square tests were used to test participant differences in STSS total scores. Primary analyses examined associations between STSS total score and subscale scores and both carotid plaque index and IMT using multinomial logistic regression models and linear regression models, respectively. Covariates (age, race/ethnicity, education, partner status, BMI, systolic blood pressure, triglycerides, LDL, HOMA, blood pressure-lowering medications, lipid-lowering medications, diabetes mellitus medications, depressive symptoms, physical activity, and dietary intake) were selected based on previously documented associations with carotid plaque or IMT, or correlations with outcomes at p < .10 in the present work, with medication variables forced into models. Secondary analyses examined any racial/ethnic differences in associations between self-silencing and carotid plaque or IMT, tested via interactions between STSS total score and subscale scores and race/ethnicity by cross-product terms in multivariable models. Significant interactions of nominal scale by continuous data were explored via the method of Aiken and West [35] using simple slopes analyses (i.e., associations between significant STSS total score or subscale scores and carotid plaque index) for white and non-white women. Analyses were performed with SAS v. 9.4 (SAS Institute) and SPSS v. 26 (IBM SPSS Statistics). Models were two-sided at α = 0.05.
Results
Participants were on average 54 years old, overweight, and normotensive. The majority of the sample was non-Hispanic white (72%) and postmenopausal (85%); see Table 1. Women with higher STSS total scores had higher depressive symptoms (r = .30, p < .001) and lower BMI (r = −.15, p = .014). STSS total scores did not differ by race (t(288) = .01, p = .989) or partner status (F(2,287) = 1.73, p = .180). Forty-seven percent (n = 136) of women had evidence of carotid plaque, and 24% (n = 70) had a plaque index ≥2.
Table 1.
Sample characteristics
| Total (N = 290) M (SD) or n (%), unless noted | |
|---|---|
| Age | 54.2 (3.9) |
| Race/ethnicity | |
| White | 210 (72.4%) |
| Black | 64 (22.1%) |
| Other | 16 (5.5%) |
| Education | |
| <College degree | 122 (42.1%) |
| ≥College degree | 168 (57.9%) |
| Menopause stage | |
| Perimenopausal | 45 (15.5%) |
| Postmenopausal | 245 (84.5%) |
| Partner status | |
| Currently partnered | 165 (56.9%) |
| Separated, divorced, widowed | 74 (25.5%) |
| Never married | 51 (17.6%) |
| Medications | |
| Antihypertensive | 46 (15.9%) |
| Antidiabetic | 9 (3.1%) |
| Lipid-lowering | 37 (12.8%) |
| BMI, kg/m2 | 29.1 (6.8) |
| SBP, mmHg | 120.2 (14.6) |
| DBP, mmHg | 70.2 (9.2) |
| LDL, mg/dL | 131.1 (33.6) |
| HDL, mg/dL | 62.7 (14.7) |
| Triglycerides, mg/dL, median (IQR) | 95.5 (71.8, 129.3) |
| HOMA, median (IQR) | 2.2 (1.7, 3.2) |
| Physical activity (IPAQ), min/week, median (IQR) | 417.0 (0.0, 1,381.5) |
| Dietary intake (FFQ), total kcal, median (IQR) | 1,219.0 (962.0, 1,565.4) |
| Depressive symptoms (CESD ≥16) | 41 (14.1%) |
| STSS total scale score and subscales | |
| STSS total score | 28.2 (8.0) |
| Self-Silencing Subscale | 7.0 (2.6) |
| Externalized Self-Perception Subscale | 6.4 (2.9) |
| Care as Self-Sacrifice Subscale | 8.4 (2.4) |
| Divided Self Subscale | 6.3 (3.3) |
| IMT, mean mm | .68 (.11) |
| Plaque index | |
| 0 | 154 (53.1%) |
| 1 | 66 (22.8%) |
| ≥2 | 70 (24.1%) |
Note. BMI body mass index; CESD Center for Epidemiologic Studies Depression scale; DBP diastolic blood pressure; FFQ Block Brief Food Frequency Questionnaire; HDL-C high-density lipoprotein cholesterol; HOMA Homeostatic Model Assessment; IMT carotid intima–media thickness; IPAQ International Physical Activity Questionnaire; IQR interquartile range; kcal kilocalories; LDL-C low-density lipoprotein cholesterol; SBP systolic blood pressure; STSS Silencing the Self Scale.
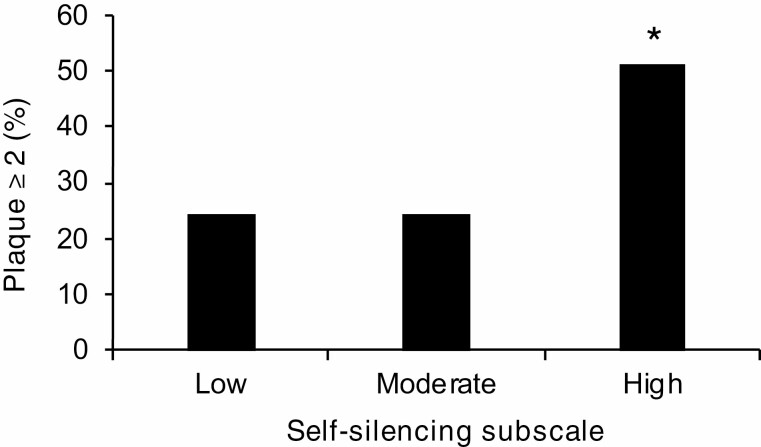
Women with higher STSS total scores and Self-Silencing Subscale scores had increased odds of carotid plaque index ≥2 after adjustment for age, race/ethnicity, education, partner status, multiple CVD risk factors, depression, physical activity, and dietary intake (Table 2). Specifically, STSS total scores were marginally related to greater plaque. Higher Self-Silencing Subscale scores were significantly related to 16% higher odds of plaque index ≥2 for each additional point on the subscale (Table 2; Fig. 1). Scores on Externalized Self-Perception, Care as Self-Sacrifice, and Divided Self Subscales were unrelated to carotid plaque index. Neither STSS total score, b(SE) = .001 (.001), p = .444, nor subscales (data not shown) were associated with IMT in fully adjusted models. Sensitivity analyses without depression did not substantively alter study findings for carotid plaque or IMT (data not shown).
Table 2.
Associations between STSS total score and subscales and carotid plaque index (N = 290)
| Plaque index | ||||
|---|---|---|---|---|
| 1 | ≥2 | |||
| STSS Total Score and Subscalesa | OR (95% CI) | p | OR (95% CI) | p |
| STSS Total Score | 1.00 (0.96–1.04) | .889 | 1.04 (1.00–1.08) | .073 |
| Self-Silencing Subscale | 0.99 (0.87–1.12) | .847 | 1.16 (1.03–1.31) | .012 |
| Externalized Self-Perception Subscale | 1.00 (0.89–1.12) | .988 | 1.05 (0.93–1.18) | .427 |
| Care as Self-Sacrifice Subscale | 0.96 (0.84–1.10) | .597 | 0.99 (0.87–1.14) | .925 |
| Divided Self Subscale | 1.02 (0.91–1.13) | .789 | 1.09 (0.98–1.20) | .105 |
Note. OR odds ratio; CI confidence interval; STSS Silencing the Self Scale.
Reference group: plaque index = 0. OR for every one-unit increase in STSS Total Score or subscale score. Covariates included age, race/ethnicity, education, partner status, body mass index, systolic blood pressure, low-density lipoprotein cholesterol, triglycerides, homeostatic model assessment, blood pressure-lowering medications, lipid-lowering medications, diabetes mellitus medications, depressive symptoms, physical activity, dietary intake.
aSTSS total score and subscale scores are considered in separate models.
Fig. 1.
Carotid plaque (index ≥ 2) by Self-Silencing Subscale scores. Note: Y-axis indicates percentage of women with plaque index ≥ 2. Self-Silencing Subscale score displayed as tertiles for illustrative purposes; Low: self-silencing subscale score 3–5; Medium: subscale score 6–7; High: subscale score ≥8. *p < .05, relative to plaque index = 0.
We further tested whether associations between STSS total score and subscale scores and carotid plaque varied by race/ethnicity (white vs. non-white [principally African American] women). STSS total scores and subscale scores did not differ by race/ethnicity (ts(288) < −.387, ps > .217). We found significant interactions between race/ethnicity and both STSS total score (plaque index ≥2, p = .016; plaque index 1, p = .646; relative to no plaque) and Self-Silencing Subscale (plaque index ≥2, p = .011; plaque index 1, p = .461; relative to no plaque) in relation to plaque; models adjusted for CVD risk factors, demographic factors, partner status, depression, physical activity, and dietary intake. To explore significant interactions, we conducted simple slope analyses of STSS total score and Self-Silencing Subscale in relation to carotid plaque. Results revealed higher STSS total scores were significantly related to 15% greater odds of carotid plaque index ≥ 2 for non-white women, but there was no significant relationship with plaque index among white women, in fully adjusted models (Table 3). Similarly, higher Self-Silencing Subscale scores were significantly related to 70% greater odds of carotid plaque index ≥2 for non-white women, but there was no significant relationship with plaque index among white women in fully adjusted models (Table 3). No significant interactions emerged between race/ethnicity and scores on Externalized Self-Perception, Care as Self-Sacrifice, or Divided Self Subscales in relation to plaque index (data not shown).
Table 3.
Associations between STSS total score and self-silencing subscale and carotid plaque index for non-white versus white women
| Plaque index | ||||||||
|---|---|---|---|---|---|---|---|---|
| Non-white women (N = 80)b | White women (N = 210) | |||||||
| 1 | ≥2 | 1 | ≥2 | |||||
| STSS scoresa | OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p |
| STSS Total Score | 1.01 (0.93–1.11) | .714 | 1.15 (1.05–1.26) | .004 | 0.99 (0.95–1.04) | .786 | 1.01 (0.97–1.06) | .550 |
| Self-Silencing Subscale | 0.90 (0.68–1.20) | .484 | 1.70 (1.22–2.38) | .002 | 1.02 (0.88–1.17) | .818 | 1.07 (0.94–1.23) | .326 |
Note. OR odds ratio; CI confidence interval; STSS Silencing the Self Scale.
Reference group: plaque index = 0. OR for every one-unit increase in STSS Total Score or Self-Silencing Subscale score. Covariates included age, education, partner status, body mass index, systolic blood pressure, low-density lipoprotein cholesterol, triglycerides, homeostatic model assessment, blood pressure-lowering medications, lipid-lowering medications, diabetes mellitus medications, depressive symptoms, physical activity, dietary intake.
aSTSS Total Score and Self-Silencing Subscale score considered in separate models. bThe majority (80%) of non-white women identified as African American.
Discussion
Using a well-characterized sample of midlife women who underwent vascular imaging, results suggest that women who report greater self-silencing of thoughts, feelings, and behaviors in their current or prior intimate relationship demonstrated greater extent of carotid atherosclerosis. Results persisted beyond adjustment for demographics, standard CVD risk factors, partner status, depressive symptoms, dietary intake, and physical activity. Further, additional analyses indicated that the relationship between self-silencing and carotid plaque index ≥2 was significant only among non-white women. No associations emerged between self-silencing and IMT.
This study was the first to examine the multidimensional construct of self-silencing with an objective measure of cardiovascular health, thus advancing the literatures on self-silencing and the social determinants of women’s cardiovascular health more broadly. In the present study, results were driven by the Self-Silencing Subscale, suggesting that the inhibition of self-expression is perhaps most potent for cardiovascular health, compared to judging oneself by external standards or caring for others as a form of self-sacrifice. Self-silencing is a strategy to maintain closeness or security in intimate relationships that are based on broader social norms regarding gender roles (e.g., women should be passive, submissive, and put their partners’ needs and feelings before their own) [36]. Women who report greater self-silencing tend to view their worth based on others’ perceptions and ultimately devalue and inhibit thoughts, feelings, or actions that they believe will jeopardize relational security or lead to rejection from their partners [16]. A variety of factors may shape self-silencing, including a person’s life experience, relationship history, social background, or emotional/power dynamics within a relationship [9, 15, 37]. Findings underscore the importance of looking beyond simple marital or partner status to consider the emotional dynamics within the relationship when considering the implications of intimate relationships for women’s cardiovascular health.
To date, only one other study has examined self-silencing and cardiovascular outcomes and showed no associations. Specifically, the Framingham Offspring Study [38], a large cohort study that includes over 1,900 women, found no association between endorsing the single-item measure of usually or always (vs. never) “self-silenced” (i.e., showed one’s feelings during conflict with their spouse) and incident coronary heart disease. However, they did find a relationship between self-silencing and increased risk of total mortality during the 10-year follow-up period. Importantly, they used a single item of self-silencing during conflict, in contrast to a multidimensional scale such as the STSS, a validated measure of self-silencing within intimate relationships.
Associations between self-silencing and vascular outcomes were observed for carotid plaque but not for IMT. The reasons for this pattern of findings are not entirely clear. IMT may reflect vascular remodeling in response to persistent hemodynamic changes (e.g., high blood pressure) [18]. Indeed, neither blood pressure nor antihypertensive medications helped explain associations with self-silencing and carotid atherosclerosis in this sample. In contrast, carotid plaque is a more direct measure of atherosclerotic burden and may be a better predictor of future CVD events for women relative to IMT [39].
Moderation analyses indicated that greater self-silencing (STSS total score and Self-Silencing Subscale) was related to significantly increased odds of carotid plaque index ≥2 only in non-white (predominantly African American) women. Although there was little difference in the odds ratios for non-white compared to white women, the significant associations with plaque index ≥2 in non-white women deserve examination in larger samples. Notably, the levels of self-silencing did not differ between racial/ethnic groups. Further, partner status did not account for racial/ethnic differences in the relation between self-silencing and carotid plaque. Our observed pattern of results is consistent with a previous study on depression that found that the relationship between self-silencing and depression was stronger in African American relative to white women [40]. It is possible that minority women, who may feel less valued or heard in society due to their gender and race/ethnicity, ultimately experience a compounding cardiovascular effect of self-silencing in both their intimate relationships and in broader society. These analyses must be viewed with caution due to their post-hoc nature and sample size limitations; however, they do underscore the importance of considering potential racial/ethnic differences when considering emotional expression and its cardiovascular sequelae among women.
The mechanisms linking self-silencing and carotid atherosclerosis are likely multiple. Standard CVD risk factors, including obesity, blood pressure, lipids, insulin resistance, and use of key medications did not explain the observed associations. By design, all women were nonsmoking. All models considered clinically elevated depressive symptoms, physical activity, and dietary intake, given evidence that women who report more self-silencing also report more depressive symptoms [9–12], and evidence that negative emotions, low physical activity, and diet quality are related to increased risk for CVD [5, 8, 41, 42]. Importantly, adjustment for depressive symptoms, physical activity, or dietary intake did not alter findings. Future work is needed to specify the stress-related physiologic pathways, such as the sympathetic nervous system or hypothalamic pituitary adrenal axis, in relations between self-silencing and carotid atherosclerosis. For example, greater cortisol and blood pressure reactivity to stress have been related to carotid atherosclerosis [43, 44], including in midlife women [45].
This study had several limitations. The observational and cross-sectional study design precludes the ability to make claims regarding directionality or causality. Details about women’s relationship histories, such as the length or timing of past/current intimate relationships, were not assessed; these factors may be important to understanding the implications of self-silencing for women’s health. Although a quarter of the sample was non-white, Asian and Hispanic women were underrepresented; the sample was largely postmenopausal; and men were not included. Thus, it is unclear if results extend to other racial/ethnic groups, to premenopausal women, or to men. Of note, the construct of self-silencing was originally developed to understand the mental health implications of gender-based expectations and perceptions of women in relationships [37]. Further, caution is warranted in interpreting results from post-hoc moderation analyses due to the relatively small sample of non-white women (and the predominance of African American women in that group). Critical next steps include replicating these findings in a larger sample of women from racial/ethnic minority backgrounds that allows for examination of relations between self-silencing and cardiovascular health across racial/ethnic groups.
This study had notable strengths. It used a well-characterized sample of midlife women and state-of-the-art measurement of carotid plaque and IMT. Analyses accounted for standard CVD risk factors and partner status, as well as depressive symptoms, physical activity, and dietary intake via widely used and well-validated measures. This study is the first to demonstrate a relationship between self-silencing and carotid atherosclerosis and advances the literature on the social determinants of women’s cardiovascular health.
Conclusions
Among midlife women, self-silencing of emotional expression was associated with carotid atherosclerosis, independent of a range of potentially confounding factors. In light of growing societal and public health interest in women’s experiences in intimate relationships, our results suggest that women’s emotional expression within intimate relationships may be relevant to their cardiovascular health. Clinicians and practitioners may be in a role to support women toward greater self-expression and to help women identify and choose healthy relationships that allow for emotional expression.
Acknowledgements
Portions of these data were presented at the North American Menopause Society 2019 Annual Meeting, Chicago, IL, USA, September 25–28, 2019.
Funding: This study was supported by the National Institutes of Health, National Heart Lung and Blood Institute (R01HL105647 and K24123565 to Dr. Thurston; T32HL082610 supporting Dr. Jakubowski), the University of Pittsburgh Clinical and Translational Science Institute (National Institutes of Health grant UL1TR000005), and the National Institute of Mental Health (T32MH018269 supporting Dr. Jakubowski).
Compliance with Ethical Standards
Authors’ Statement of Conflict of Interest and Adherence to Ethical Standards Authors Dr. Maki has served as a consultant for Balchem, Pfizer, and AbbVie. The remaining authors declare that they have no conflict of interest.
Authors’ Contributions:
All authors met the criteria for authorship: (1) Substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; (2) Drafting and/or critically revising the article for important intellectual content; and (3) Final approval of the version to be published and agreement to be accountable for the integrity and accuracy of all aspects of the work.
Ethical Approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The study was approved by the University of Pittsburgh Institutional Review Board.
Informed Consent: Informed consent was obtained from all individual participants included in the study.
References
- 1. Holt-Lunstad J, Smith TB, Baker M, Harris T, Stephenson D. Loneliness and social isolation as risk factors for mortality: a meta-analytic review. Perspect Psychol Sci. 2015;10:227–237. [DOI] [PubMed] [Google Scholar]
- 2. Hakulinen C, Pulkki-Råback L, Virtanen M, Jokela M, Kivimäki M, Elovainio M. Social isolation and loneliness as risk factors for myocardial infarction, stroke and mortality: UK Biobank cohort study of 479 054 men and women. Heart. 2018;104:1536–1542. [DOI] [PubMed] [Google Scholar]
- 3. Laugesen K, Baggesen LM, Schmidt SAJ, et al. Social isolation and all-cause mortality: a population-based cohort study in Denmark. Sci Rep. 2018;8:4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kiecolt-Glaser JK, Newton TL. Marriage and health: his and hers. Psychol Bull. 2001;127:472–503. [DOI] [PubMed] [Google Scholar]
- 5. Benjamin EJ, Muntner P, Alonso A, et al. ; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee . Heart Disease and Stroke Statistics-2019 Update: a report from the American Heart Association. Circulation. 2019;139:e56–e528. [DOI] [PubMed] [Google Scholar]
- 6. Jack DC. Silencing the Self: Women and Depression. Cambridge, MA: Harvard University Press; 1991. [Google Scholar]
- 7. O’Neil A, Scovelle AJ, Milner AJ, Kavanagh A. Gender/sex as a social determinant of cardiovascular risk. Circulation. 2018;137:854–864. [DOI] [PubMed] [Google Scholar]
- 8. Low CA, Thurston RC, Matthews KA. Psychosocial factors in the development of heart disease in women: current research and future directions. Psychosom Med. 2010;72:842–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jack DC, Dill D. The Silencing the Self Scale: schemas of intimacy associated with depression in women. Psychol Women Q. 1992;16:97–106. [Google Scholar]
- 10. Cramer KM, Gallant MD, Langlois MW. Self-silencing and depression in women and men: comparative structural equation models. Pers Individ Dif. 2005;39:581–592. [Google Scholar]
- 11. Grant TM, Jack DC, Fitzpatrick AL, Ernst CC. Carrying the burdens of poverty, parenting, and addiction: depression symptoms and self-silencing among ethnically diverse women. Community Ment Health J. 2011;47:90–98. [DOI] [PubMed] [Google Scholar]
- 12. Ussher JM, Perz J. Gender differences in self-silencing and psychological distress in informal cancer carers. Psychol Women Q. 2010;34:228–242. [Google Scholar]
- 13. Buchholz A, Henderson KA, Hounsell A, Wagner A, Norris M, Spettigue W. Self-silencing in a clinical sample of female adolescents with eating disorders. J Can Acad Child Adolesc Psychiatry. 2007;16:158–163. [PMC free article] [PubMed] [Google Scholar]
- 14. Norwood SJ, Bowker A, Buchholz A, Henderson KA, Goldfield G, Flament MF. Self-silencing and anger regulation as predictors of disordered eating among adolescent females. Eat Behav. 2011;12:112–118. [DOI] [PubMed] [Google Scholar]
- 15. Ali A, Toner BB, Stuckless N, et al. Emotional abuse, self-blame, and self-silencing in women with irritable bowel syndrome. Psychosom Med. 2000;62:76–82. [DOI] [PubMed] [Google Scholar]
- 16. Maji S, Dixit S. Self-silencing and women’s health: a review. Int J Soc Psychiatry. 2019;65:3–13. [DOI] [PubMed] [Google Scholar]
- 17. Mehta LS, Beckie TM, DeVon HA, et al. Acute myocardial infarction in women: a scientific statement from the American Heart Association. Circulation. 2016;133:916–947. [DOI] [PubMed] [Google Scholar]
- 18. Stein JH, Korcarz CE, Hurst RT, et al. Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: a consensus statement from the American Society of Echocardiography Carotid Intima-Media Thickness Task Force. Endorsed by the Society for Vascular Medicine. J Am Soc Echocardiogr. 2008;21:93–111; quiz 189–190. [DOI] [PubMed] [Google Scholar]
- 19. Polak JF, Szklo M, Kronmal RA, et al. The value of carotid artery plaque and intima-media thickness for incident cardiovascular disease: the multi-ethnic study of atherosclerosis. J Am Heart Assoc. 2013;2:e000087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Peters SA, den Ruijter HM, Bots ML, Moons KG. Improvements in risk stratification for the occurrence of cardiovascular disease by imaging subclinical atherosclerosis: a systematic review. Heart. 2012;98:177–184. [DOI] [PubMed] [Google Scholar]
- 21. Thurston RC, Chang Y, Barinas-Mitchell E, et al. Menopausal hot flashes and carotid intima media thickness among midlife women. Stroke. 2016;47:2910–2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sutton-Tyrrell K, Wolfson SK Jr, Thompson T, Kelsey SF. Measurement variability in duplex scan assessment of carotid atherosclerosis. Stroke. 1992;23:215–220. [DOI] [PubMed] [Google Scholar]
- 23. Wendelhag I, Gustavsson T, Suurküla M, Berglund G, Wikstrand J. Ultrasound measurement of wall thickness in the carotid artery: fundamental principles and description of a computerized analysing system. Clin Physiol. 1991;11:565–577. [DOI] [PubMed] [Google Scholar]
- 24. Thompson T, Sutton-Tyrrell K, Wildman RP, et al. Progression of carotid intima-media thickness and plaque in women with systemic lupus erythematosus. Arthritis Rheum. 2008;58:835–842. [DOI] [PubMed] [Google Scholar]
- 25. Touboul PJ, Hennerici MG, Meairs S, et al. Mannheim carotid intima-media thickness and plaque consensus (2004-2006-2011). An update on behalf of the advisory board of the 3rd, 4th and 5th watching the risk symposia, at the 13th, 15th and 20th European Stroke Conferences, Mannheim, Germany, 2004, Brussels, Belgium, 2006, and Hamburg, Germany, 2011. Cerebrovasc Dis. 2012;34:290–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sutton-Tyrrell K, Kuller LH, Matthews KA, et al. Subclinical atherosclerosis in multiple vascular beds: an index of atherosclerotic burden evaluated in postmenopausal women. Atherosclerosis. 2002;160:407–416. [DOI] [PubMed] [Google Scholar]
- 27. Harlow SD, Gass M, Hall JE, et al. ; STRAW + 10 Collaborative Group . Executive summary of the Stages of Reproductive Aging Workshop + 10: addressing the unfinished agenda of staging reproductive aging. J Clin Endocrinol Metab. 2012;97:1159–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Radloff LS. The CES-D Scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 29. Craig CL, Marshall AL, Sjöström M, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35:1381–1395. [DOI] [PubMed] [Google Scholar]
- 30. Block G, Hartman AM, Naughton D. A reduced dietary questionnaire: development and validation. Epidemiology. 1990;1:58–64. [DOI] [PubMed] [Google Scholar]
- 31. Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 32. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. [DOI] [PubMed] [Google Scholar]
- 33. Radler BT, Rigotti A, Ryff CD. Persistently high psychological well-being predicts better HDL cholesterol and triglyceride levels: findings from the midlife in the U.S. (MIDUS) longitudinal study. Lipids Health Dis. 2018;17:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kim TH, Carroll JE, An SK, Seeman TE, Namkoong K, Lee E. Associations between actigraphy-assessed sleep, inflammatory markers, and insulin resistance in the Midlife Development in the United States (MIDUS) study. Sleep Med. 2016;27–28:72–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Aiken SG, West LS.. Multiple Regression: Testing and Interpreting Interactions. Newbury Park, CA: Sage; 1991. [Google Scholar]
- 36. Jack DC, Ali A, eds. Silencing the Self across Cultures: Depression and Gender in the Social World. Oxford: Oxford University Press; 2010. [Google Scholar]
- 37. Jack DC. Reflections on the silencing the self scale and its origins. Psychol Women Q. 2011;35:523–529. [Google Scholar]
- 38. Eaker ED, Sullivan LM, Kelly-Hayes M, D’Agostino RB Sr, Benjamin EJ. Marital status, marital strain, and risk of coronary heart disease or total mortality: the Framingham Offspring Study. Psychosom Med. 2007;69:509–513. [DOI] [PubMed] [Google Scholar]
- 39. Naqvi TZ, Lee MS. Carotid intima-media thickness and plaque in cardiovascular risk assessment. JACC Cardiovasc Imaging. 2014;7:1025–1038. [DOI] [PubMed] [Google Scholar]
- 40. Gratch LV, Bassett ME, Attra SL. The relationship of gender and ethnicity to self-silencing and depression among college students. Psychol Women Q. 1995;19:509–515. [Google Scholar]
- 41. O’Neil A, Fisher AJ, Kibbey KJ, et al. Depression is a risk factor for incident coronary heart disease in women: an 18-year longitudinal study. J Affect Disord. 2016;196:117–124. [DOI] [PubMed] [Google Scholar]
- 42. Li J, Siegrist J. Physical activity and risk of cardiovascular disease – a meta-analysis of prospective cohort studies. Int J Environ Res Public Health. 2012;9:391–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Matthews KA, Zhu S, Tucker DC, Whooley MA. Blood pressure reactivity to psychological stress and coronary calcification in the Coronary Artery Risk Development in Young Adults Study. Hypertension. 2006;47:391–395. [DOI] [PubMed] [Google Scholar]
- 44. Hamer M, Endrighi R, Venuraju SM, Lahiri A, Steptoe A. Cortisol responses to mental stress and the progression of coronary artery calcification in healthy men and women. PLoS One. 2012;7:e31356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Matthews KA, Owens JF, Kuller LH, et al. Stress-induced pulse pressure change predicts women’s carotid atherosclerosis. Stroke. 1998;29:1525–1530. [DOI] [PubMed] [Google Scholar]