Abstract
BACKGROUND
Prophylactic use of tranexamic acid at the time of cesarean delivery has been shown to decrease the calculated blood loss, but the effect on the need for blood transfusions is unclear.
METHODS
We randomly assigned patients undergoing cesarean delivery at 31 U.S. hospitals to receive either tranexamic acid or placebo after umbilical-cord clamping. The primary outcome was a composite of maternal death or blood transfusion by hospital discharge or 7 days post partum, whichever came first. Key secondary outcomes were estimated intraoperative blood loss of more than 1 liter (prespecified as a major secondary outcome), interventions for bleeding and related complications, the preoperative-to-postoperative change in the hemoglobin level, and postpartum infectious complications. Adverse events were assessed.
RESULTS
A total of 11,000 participants underwent randomization (5529 to the tranexamic acid group and 5471 to the placebo group); scheduled cesarean delivery accounted for 50.1% and 49.2% of the deliveries in the respective groups. A primary-outcome event occurred in 201 of 5525 participants (3.6%) in the tranexamic acid group and in 233 of 5470 (4.3%) in the placebo group (adjusted relative risk, 0.89; 95.26% confidence interval [CI], 0.74 to 1.07; P = 0.19). Estimated intraoperative blood loss of more than 1 liter occurred in 7.3% of the participants in the tranexamic acid group and in 8.0% of those in the placebo group (relative risk, 0.91; 95% CI, 0.79 to 1.05). Interventions for bleeding complications occurred in 16.1% of the participants in the tranexamic acid group and in 18.0% of those in the placebo group (relative risk, 0.90; 95% CI, 0.82 to 0.97); the change in the hemoglobin level was −1.8 g per deciliter and −1.9 g per deciliter, respectively (mean difference, −0.1 g per deciliter; 95% CI, −0.2 to −0.1); and postpartum infectious complications occurred in 3.2% and 2.5% of the participants, respectively (relative risk, 1.28; 95% CI, 1.02 to 1.61). The frequencies of thromboembolic events and other adverse events were similar in the two groups.
CONCLUSIONS
Prophylactic use of tranexamic acid during cesarean delivery did not lead to a significantly lower risk of a composite outcome of maternal death or blood transfusion than placebo. (Funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development; ClinicalTrials.gov number, NCT03364491.)
Postpartum hemorrhage is responsible for up to 27.1% of maternal deaths worldwide.1 In the United States, obstetrical hemorrhage is the second most common cause of pregnancy-related death, second only to cardiovascular disease.2 Among patients with bleeding trauma, the use of tranexamic acid, a fibrinolysis inhibitor, was associated with a significant reduction in overall mortality.3 Similar results have been reported in patients with traumatic brain injury and established obstetrical hemorrhage.4,5
An increase in fibrinolytic activity (as indicated by increased levels of tissue plasminogen activator and D-dimer) after the delivery of the placenta has been previously described.6 It is biologically plausible that fibrinolysis inhibition with the use of tranexamic acid after delivery could improve hemostasis by preventing clot breakdown. The use of tranexamic acid to prevent obstetrical hemorrhage has been reported previously.7,8 However, most studies of postpartum hemorrhage have been single-center trials that have been limited by small sample sizes with a lack of power to evaluate important clinical outcomes.
Two large, multicenter clinical trials addressed the use of tranexamic acid to prevent hemorrhage after either vaginal or cesarean delivery.9,10 Prophylactic use of tranexamic acid did not reduce the incidence of postpartum hemorrhage among patients delivering vaginally. In patients undergoing cesarean delivery, the use of tranexamic acid decreased the estimated calculated blood loss by a mean of approximately 100 ml. There were no benefits with regard to other clinically significant outcomes such as transfusion of blood products or the use of additional surgical or radiologic interventions to control bleeding in either trial. Moreover, more than 70% of the participants in the cesarean trial underwent delivery in the absence of labor and thus were at lower risk for hemorrhage than participants who underwent cesarean delivery after the onset of labor. Neither of these two previous trials were powered to detect a difference in the incidence of transfusion of blood products. We designed a randomized trial to evaluate whether the administration of tranexamic acid immediately after umbilical-cord clamping would lead to a lower risk of maternal death or blood transfusion than placebo among patients undergoing scheduled or emergency cesarean delivery.
METHODS
Trial Oversight
We designed and conducted this multicenter, double-blind, randomized, controlled trial at 31 hospitals participating in the Maternal–Fetal Medicine Units Network of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD). The protocol (which is available with the full text of this article at NEJM.org) was approved by the institutional review board at each hospital before the enrollment of participants. Written informed consent was obtained from all the participants before randomization. An independent data and safety monitoring committee that was appointed by the NICHD monitored the trial. The first four authors vouch for the accuracy and completeness of the data and for the fidelity of the trial to the protocol. An Investigational New Drug Application was approved by the Food and Drug Administration for the use of prophylactic tranexamic acid in this trial.
SCREENING AND RECRUITMENT
All the patients who were admitted to the labor and delivery unit for planned delivery were eligible for screening. Patients who had a scheduled cesarean delivery could also be screened in the outpatient setting. After admission, patients who met the trial criteria were informed about the trial and were given the opportunity to provide informed consent to participate by trained research personnel. Patients who were planning to deliver vaginally signed the informed consent form with the understanding that they would not undergo randomization unless they underwent a cesarean delivery.
Patients were eligible if they had a scheduled or unscheduled cesarean delivery of a singleton or twin gestation. Key exclusion criteria were a maternal age of less than 18 years, the transfusion of any blood products before randomization during the current admission or a plan for transfusion after randomization, contraindications to tranexamic acid (e.g., hypersensitivity to tranexamic acid or its ingredients and a history of seizure disorder, kidney disease, thromboembolic disease, or medical conditions or treatments associated with a high risk of thrombosis), the patient’s decision to not use blood products, or the existence of a plan to administer prophylactic antifibrinolytic agents such as open-label tranexamic acid or uterotonic agents other than oxytocin preoperatively.
RANDOMIZATION AND TREATMENT STRATEGY
Patients who provided informed consent to participate and who were undergoing cesarean delivery were randomly assigned in a 1:1 ratio to receive either 1 g (10 ml) of tranexamic acid diluted in 40 ml of normal saline or identically appearing placebo (50 ml of normal saline) intravenously over a period of 10 minutes immediately after umbilical-cord clamping. The randomization sequence was prepared by an independent data coordinating center and followed the simple randomization method with stratification according to clinical site. Tranexamic acid and placebo were prepared by the local research pharmacy. None of the participants, investigators, or clinical staff were aware of the trial-group assignments.
Randomization was restricted to allow a maximum of 50% scheduled cesarean deliveries as compared with unscheduled cases; however, this classification was not used for stratification. Scheduled cesarean delivery was defined as the participant appearing on the schedule for cesarean delivery on the day of randomization, with no evidence of labor or rupture of membranes. All the other aspects of clinical care were left to the discretion of the treating providers. If open-label tranexamic acid was used because of excessive intraoperative bleeding, it was recommended that its use be delayed for at least 30 minutes after the administration of the trial infusion. No more than two doses total (trial infusion plus one dose of open-label tranexamic acid) were allowed during the first 24 hours after delivery.
Trial Outcomes
The primary outcome was a composite of maternal death or blood transfusion before discharge from the hospital or 7 days post partum, whichever occurred first. Blood transfusion was defined as the transfusion of packed red cells or whole blood or the use of a cell-saver autotransfusion device.
Estimated intraoperative blood loss of more than 1 liter, assessed on the basis of data obtained from the anesthesia record and the operative report, was a major secondary outcome. Key secondary outcomes (which were prespecified as such once the trial was ongoing but before any data were analyzed) included a composite of treatments and interventions in response to bleeding and related complications within 7 days post partum (i.e., surgical or radiologic procedures to control bleeding and related complications [e.g., laparotomy, evacuation of hematoma, hysterectomy, uterine packing, intrauterine balloon tamponade, or interventional radiologic procedures], use of uterotonic agents other than oxytocin, use of open-label tranexamic acid or any other antifibrinolytic agent, or transfusion of any blood product); infectious complications (endometritis, surgical-site infection, or pelvic abscess) within 6 weeks post partum; the change in the preoperative hemoglobin level (most recent value obtained ≤4 weeks before delivery) as compared with the lowest postoperative value (obtained ≤48 hours after delivery); and maternal thromboembolic events (including venous, arterial, or ischemic stroke or myocardial infarction). Adverse events were also assessed. Details of the other prespecified secondary efficacy and safety outcomes are provided in Table S1 in the Supplementary Appendix, available at NEJM.org.
Trained and certified research staff members, who were unaware of the trial-group assignments, abstracted information from medical records, including demographic information, medical history, and outcomes data. Participants were interviewed by research personnel at 1 to 2 weeks post partum and again at 6 weeks post partum. During this interview, participants were asked about any hospital admissions since discharge, emergency department visits, and the development of adverse events, including thrombotic events.
STATISTICAL ANALYSIS
On the basis of data from the Maternal–Fetal Medicine Units Network Cesarean Registry and the Observational Cohort Study to Evaluate Measures of Quality of Obstetric Care (APEX),11,12 we expected that the risk of a primary-outcome event in the placebo group would be 2.5%. We calculated that the enrollment of 11,000 participants (5500 per group) would provide the trial with 85% power to detect a 33% lower risk of the primary outcome (i.e., 1.68%) in the tranexamic acid group at a two-sided type I error of 5%. Two interim analyses with the use of the Lan–DeMets generalization of the O’Brien–Fleming boundary were conducted and reviewed by the data and safety monitoring committee. In the final analysis of the primary outcome, a two-tailed P value of less than 0.047 was considered to indicate statistical significance.
Analyses were performed according to the intention-to-treat principle; the intention-to-treat population included all the participants who had undergone randomization, with the exception of those who had been enrolled during a previous pregnancy. The safety population included all the participants who received tranexamic acid or placebo, according to the treatment they actually received. Baseline characteristics and selected secondary outcomes were compared by means of the chi-square test, Fisher’s exact test, or the Wilcoxon rank-sum test as appropriate. The primary outcome was compared with the use of a log-binomial regression model. If the trial groups were found to differ on a pretreatment factor that was known to be a risk factor for the outcome, the statistical analysis was adjusted for these differences. Log-binomial regression was used to calculate the adjusted relative risks and confidence intervals. Exact methods for Poisson regression were used to compute confidence intervals for rare binary end points. A sensitivity analysis of the primary outcome that excluded all the participants who received open-label tranexamic acid was conducted. In accordance with the protocol, prespecified subgroup analyses were conducted only if there was a significant between-group difference in the primary outcome. Changes in the preoperative-to-postoperative hemoglobin level were compared with a generalized linear model. Two sensitivity analyses were conducted for missing data: for participants who had missing data on the estimated intraoperative blood loss, data on quantitative blood loss were used; and for participants who had missing data on the postoperative hemoglobin level, the postoperative hematocrit divided by 3 was used in the calculation of the change in the hemoglobin level.
Adjustment of the P value for the major secondary outcome was planned if the results of the primary-outcome analysis were significant. No other secondary outcome results were adjusted for multiplicity, and no formal inferences or conclusions can be drawn from the other results. The statistical analysis plan is provided with the protocol.
RESULTS
TRIAL PARTICIPANTS
From March 2018 through July 2021, a total of 84,062 patients underwent screening for randomization, of whom 44,574 were ineligible and 28,488 declined to participate. Thus, 11,000 patients underwent randomization: 5529 participants were assigned to the tranexamic acid group, and 5471 to the placebo group (Fig. 1). Five patients presented with two different pregnancies during the trial period; only the first pregnancy was included in the analysis, which resulted in 5525 participants in the tranexamic acid group and 5470 in the placebo group.
Figure 1. Eligibility, Randomization, and Assessment of the Participants.
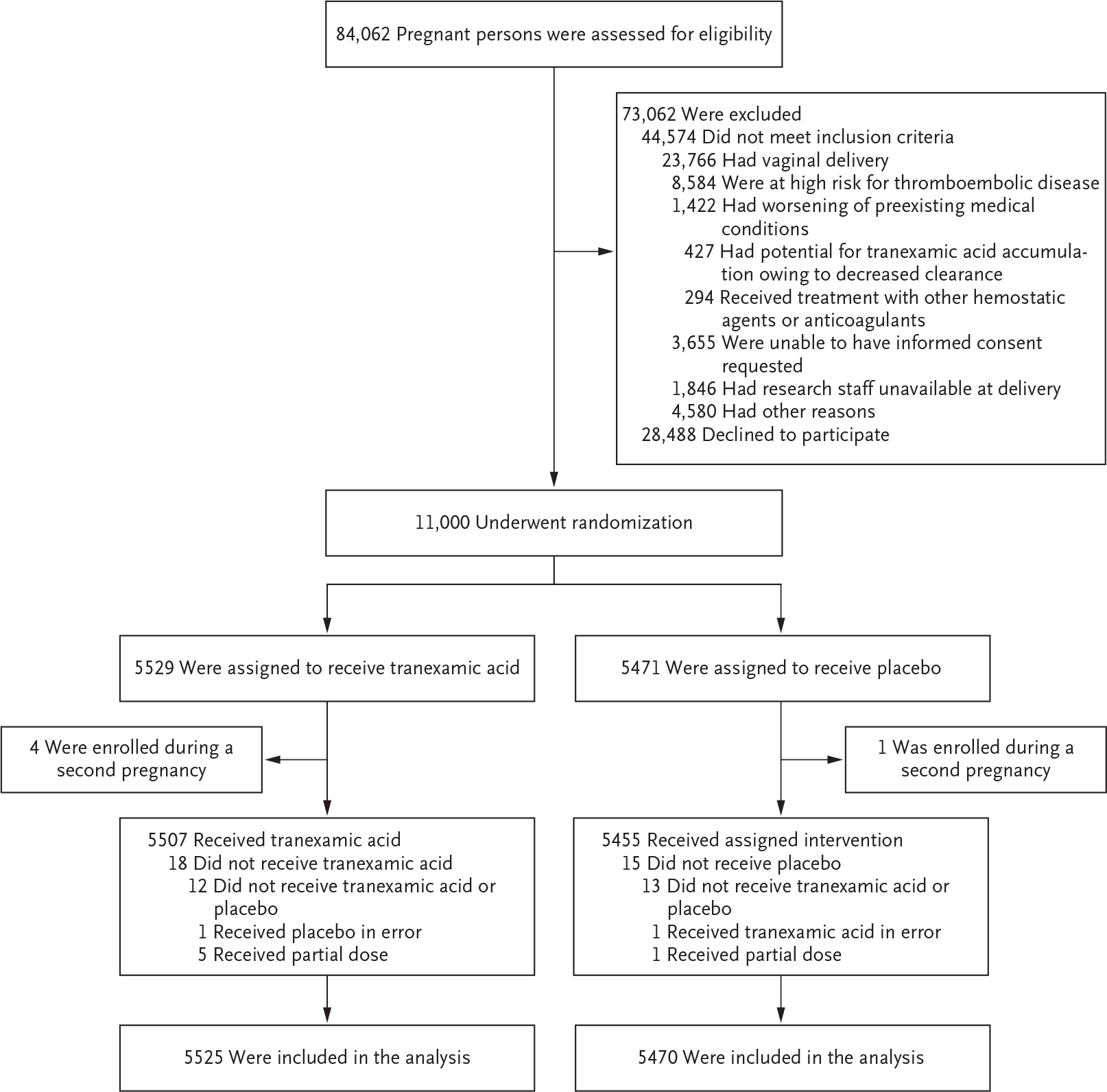
Five participants (4 in the tranexamic acid group and 1 in the placebo group) were enrolled during two different pregnancies during the trial period. Only the first pregnancy was included in the analysis, which resulted in 5525 participants in the tranexamic acid group and 5470 in the placebo group.
The characteristics of the participants at baseline did not differ substantially between the two groups except for the preoperative hemoglobin level; a level below 8 g per deciliter was more common in the placebo group than in the tranexamic acid group (Table 1). Scheduled cesarean delivery accounted for 2768 of the cesarean deliveries (50.1%) in the tranexamic acid group and for 2693 of those (49.2%) in the placebo group. Tranexamic acid or placebo was administered within 3 minutes after umbilical-cord clamping in 91.8% of the participants.
Table 1.
Maternal Characteristics.*
| Characteristic | Tranexamic Acid (N=5525) | Placebo (N = 5470) |
|---|---|---|
| Maternal age — yr | 30.1±5.8 | 30.1±5.8 |
| Race or ethnic group — no. (%)† | ||
| Non-Hispanic White | 2170 (39.3) | 2159 (39.5) |
| Non-Hispanic Black | 1334 (24.1) | 1310 (23.9) |
| Hispanic | 1636 (29.6) | 1642 (30.0) |
| Asian | 218 (3.9) | 193 (3.5) |
| Other, unknown, or multiple | 167 (3.0) | 166 (3.0) |
| Body-mass index at deliver‡ | ||
| Mean | 35.7+8.2 | 35.7+8.2 |
| ≥30 — no./total. no. (%) | 4099/5503 (74.5) | 4031/5459 (73.8) |
| Nulliparous — no. (%) | 1660 (30.0) | 1713 (31.3) |
| Placenta previa — no. (%) | 92 (1.7) | 104 (1.9) |
| Twin pregnancy — no. (%) | 234 (4.2) | 224 (4.1) |
| Both twins delivered by cesarean section — no./total no. (%) | 231/234 (98.7) | 216/224 (96.4) |
| Pregnancy-related hypertensive disorder — no. (%) | 957 (17.3) | 967 (17.7) |
| Previous cesarean delivery — no. (%) | 3150 (57.0) | 3026 (55.3) |
| Scheduled cesarean delivery — no. (%) | 2768 (50.1) | 2693 (49.2) |
| Placental abruption — no. (%) | 43 (0.8) | 44 (0.8) |
| Type of labor — no. (%) | ||
| Spontaneous | 467 (8.5) | 436 (8.0) |
| Spontaneous with augmentation | 242 (4.4) | 248 (4.5) |
| Induced | 1282 (23.2) | 1337 (24.4) |
| None | 3534 (64.0) | 3449 (63.1) |
| Arrest of labor — no. (%) | ||
| Stage 1 | 660 (11.9) | 708 (12.9) |
| Stage 2 | 175 (3.2) | 192 (3.5) |
| None or no labor§ | 4690 (84.9) | 4570 (83.5) |
| Oxytocin during labor — no. (%) | 1466 (26.5) | 1517 (27.7) |
| Placenta accreta, increta, or percreta — no. (%) | 16 (0.3) | 17 (0.3) |
| Infant birth weight >4000 g — no./total no. (%) | 478/5524 (8.7) | 453/5470 (8.3) |
| Chorioamnionitis — no. (%) | 182 (3.3) | 184 (3.4) |
| Gestational age at deliver¶ | ||
| Mean — wk | 38.3+2.0 | 38.3+2.0 |
| Distribution — no. (%) | ||
| <34 wk | 143/5524 (2.6) | 151/5470 (2.8) |
| 34–36 wk | 693/5524 (12.5) | 689/5470 (12.6) |
| ≥37 wk | 4688/5524 (84.9) | 4630/5470 (84.6) |
| Preoperative hemoglobin level <8 g/dl — no./total no. (%) | 10/5523 (0.2) | 26/5464 (0.5) |
| Administration of tranexamic acid or placebo within 3 min after umbilical-cord clamping — no./total no. (%)‖ | 5049/5511 (91.6) | 5013/5455 (91.9) |
Plus–minus values are means ±SD. All the characteristics were similar (P>0.05) in the two groups except for a preoperative hemoglobin level of less than 8 g per deciliter (P = 0.007). Percentages may not total 100 because of rounding.
Race and ethnic group were reported by the participant.
The body-mass index is the weight in kilograms divided by the square of the height in meters. Data were missing for 22 participants in the tranexamic acid group and for 11 in the placebo group.
“None” included participants with labor who underwent cesarean delivery for indications other than labor arrest.
Data were missing for one participant in the tranexamic acid group.
Data were missing for 2 participants who received tranexamic acid and for 2 who received placebo because the time of starting tranexamic acid or placebo or the time of umbilical-cord clamping was not obtained. Data were missing for 12 participants in the tranexamic group and for 13 in the placebo group because neither tranexamic acid nor placebo was received.
OUTCOMES
A primary-outcome event occurred in 3.6% of the participants in the tranexamic acid group and in 4.3% of those in the placebo group (adjusted relative risk, 0.89; 95.26% confidence interval [CI], 0.74 to 1.07; P = 0.19) (Table 2). We did not observe material differences according to trial center. After the exclusion of participants who received open-label tranexamic acid (108 in the tranexamic acid group and 109 in the placebo group), the adjusted relative risk was 0.84 (95.26% CI, 0.68 to 1.03).
Table 2.
Primary and Secondary Outcomes.*
| Outcome | Tranexamic Acid (N =5525) | Placebo (N = 5470) | Relative Risk or Mean Difference (95% Cl)† |
|---|---|---|---|
| Primary outcome: maternal death or blood transfusion by hospital discharge or 7 days post partum, whicheverwas earlier— no. (%) | 201 (3.6) | 233 (4.3) | 0.89 (0.74 to 1.07)‡ |
| Maternal death | 0 | 1 (<0.1) | — |
| Blood transfusion | 201 (3.6) | 232 (4.2) | 0.86 (0.71 to 1.03) |
| Estimated blood loss >1 liter— no./total no. (%) | 339/4641 (7.3) | 368/4573 (8.0) | 0.91 (0.79 to 1.05) |
| Intervention in response to bleeding and related complications by 7 days post partum — no. (%) | 892 (16.1) | 986 (18.0) | 0.90 (0.82 to 0.97) |
| Surgical or radiologic intervention by 7 days post partum — no. (%) | 233 (4.2) | 231 (4.2) | 1.00 (0.84 to 1.19) |
| Uterotonic agent other than oxytocin by 48 hr post partum — no. (%) | 649 (11.7) | 732 (13.4) | 0.88 (0.80 to 0.97) |
| Open-label use of tranexamic acid by 7 days post partum — no. (%) | 108 (2.0) | 109 (2.0) | 0.98 (0.75 to 1.28) |
| Transfusion of any blood product by 7 days post partum — no. (%) | 205 (3.7) | 238 (4.4) | 0.85 (0.71 to 1.02) |
| Change in hemoglobin level — g/dl§ | −1.8+1.1 | −1.9+1.1 | −0.1 (−0.2 to −0.1) |
| Transfusion of blood products other than packed red cells by 7 days post partum — no. (%) | 29 (0.5) | 31 (0.6) | 0.93 (0.56 to 1.53) |
| Blood transfusion of >4 units by 7 days post partum — no. (%) | 20 (0.4) | 19 (0.3) | 1.04 (0.56 to 1.95) |
| Median postoperative duration of hospital stay (IQ R) — days | 3 (2 to 3) | 3 (2 to 3) | 0.0 (−0.1 to 0.0) |
| Acute kidney injury by 7 days post partum — no. (%) | 30 (0.5) | 27 (0.5) | 1.10 (0.65 to 1.85) |
| Transfusion-associated reaction by 7 days post partum — no. (%) | 5 (0.1) | 3 (0.1) | 1.65 (0.32 to 10.63) |
| Postpartum infectious complication by 6 wk — no./total no. (%) | 162/5080 (3.2) | 125/5009 (2.5) | 1.28 (1.02 to 1.61) |
| Endometritis | 54/5080 (1.1) | 42/5009 (0.8) | 1.27 (0.85 to 1.89) |
| Surgical-site infection | 104/5080 (2.0) | 81/5009 (1.6) | 1.27 (0.95 to 1.69) |
| Pelvic abscess | 7/5080 (0.1) | 3/5009 (0.1) | 2.30 (0.53 to 13.8) |
Blood transfusion was defined as the transfusion of packed red cells or whole blood or use of a cell-saver autotransfusion device. IQR denotes interquartile range.
Relative risks are provided for analyses in which numbers and percentages of participants are reported, and mean differences for analyses in which mean or median values are reported. The primary-outcome analysis was adjusted for a preoperative hemoglobin level of less than 8 g per deciliter and used a 95.26% confidence interval (on the basis of a P-value threshold of less than 0.047). All the other analyses were unadjusted and used 95% confidence intervals.
P = 0.19.
The change in hemoglobin level was assessed by comparing the most recent value obtained within 4 weeks before delivery to the lowest measurement obtained during the first 48 hours post partum. Data were available for 5224 participants in the tranexamic acid group and for 5201 in the placebo group.
Estimated intraoperative blood loss of more than 1 liter occurred in 7.3% of the participants in the tranexamic acid group and in 8.0% of those in the placebo group (relative risk, 0.91; 95% CI, 0.79 to 1.05). The composite of treatments and interventions in response to bleeding and related complications occurred in 16.1% of the participants in the tranexamic acid group and in 18.0% of those in the placebo group (relative risk, 0.90; 95% CI, 0.82 to 0.97). The mean preoperative-to-postoperative change in the hemoglobin level was −1.8 g per deciliter in the tranexamic acid group and −1.9 g per deciliter in the placebo group (mean difference, −0.1 g per deciliter; 95% CI, −0.2 to −0.1). Postpartum infectious complications occurred in 3.2% of the participants in the tranexamic acid group and in 2.5% of those in the placebo group (relative risk, 1.28; 95% CI, 1.02 to 1.61). Other secondary outcomes are summarized in Table 2. In sensitivity analyses that accounted for missing data (estimated blood loss and change in the hemoglobin level), the results were similar to those in the main analysis (Tables S3 and S4). There were no material between-group differences in the incidence of major safety outcomes, including thromboembolic events or new-onset seizure activity (Table 3).
Table 3.
Safety Outcomes.*
| Event | Tranexamic Acid (N = 5513) | Placebo (N = 5457) | Relative Risk (95% Cl) | P Value |
|---|---|---|---|---|
| Thromboembolic event, ischemic stroke, or myocardial infarction — no./total no. (%) | 12/5069 (0.2) | 13/4996 (0.3) | 0.91 (0.42–1.99) | 0.81 |
| Thromboembolic event, venous or arterial† | 8/5069 (0.2) | 13/4996 (0.3) | 0.61 (0.25–1.46) | 0.26 |
| Ischemic stroke | 2/5069 (<0.1) | 0/4996 | — | 0.50 |
| Myocardial infarction | 2/5069 (<0.1) | 0/4996 | — | 0.50 |
| New-onset seizure — no./total no. (%) | 2/5069 (<0.1) | 0/4996 | — | 0.50 |
| Admission to ICU for more than 24 hr— no./total no. (%) | 21/5069 (0.4) | 17/4996 (0.3) | 1.22 (0.64–2.30) | 0.55 |
| Maternal death — no./total no. (%)‡ | 2/5069 (<0.1) | 2/4996 (<0.1) | 0.99 (0.07–13.6) | >0.99 |
| Thromboembolic event, ischemic stroke, myocardial infarction, new-onset seizure activity, admission to the ICU for more than 24 hr, or maternal death — no./total no. (%) | 35/5069 (0.7) | 32/4996 (0.6) | 1.08 (0.67–1.74) | 0.76 |
| Hospital readmission — no./total no. (%) | 199/5069 (3.9) | 162/4996 (3.2) | 1.21 (0.99–1.48) | 0.07 |
| Any side effect— no. (%)§ | 616 (11.2) | 667 (12.2) | 0.91 (0.82–1.01) | 0.09 |
| Nausea | 362 (6.6) | 403 (7.4) | 0.89 (0.78–1.02) | 0.09 |
| Vomiting | 266 (4.8) | 273 (5.0) | 0.96 (0.82–1.14) | 0.67 |
| Dizziness | 156 (2.8) | 186 (3.4) | 0.83 (0.67–1.02) | 0.08 |
The safety population included all the participants who received tranexamic acid or placebo, according to the treatment they actually received. Risks of thromboembolic event, ischemic stroke, myocardial infarction, new-onset seizure, admission to intensive care unit (ICU) for more than 24 hours, maternal death, and hospital readmission were assessed until 6 weeks post partum.
All the thromboembolic events that occurred were venous.
Of the four deaths, one (in the placebo group) occurred 1 day after delivery and was counted as part of the primary outcome (cause of death was undetermined). The other three deaths occurred after discharge, and the causes were septic shock of fungal cause (in the tranexamic acid group), trauma-induced injury (in the tranexamic acid group), and opioid overdose (in the placebo group).
The analysis of side effects included all the events that occurred by 24 hours post partum.
DISCUSSION
In this randomized trial involving patients undergoing either scheduled or unscheduled cesarean delivery, the prophylactic use of intravenous tranexamic acid immediately after umbilical-cord clamping did not lead to a significantly lower risk of the composite outcome of maternal death or blood transfusion. We did not find a material between-group difference in the incidence of estimated intraoperative blood loss of more than 1 liter.
Treatments and surgical interventions in response to bleeding and related complications (composite secondary outcome) occurred in 16.1% of the participants in the tranexamic acid group and in 18.0% of those in the placebo group (Table S2). Although our findings suggest the possibility that prophylactic tranexamic acid may have led to a lower risk of interventions in response to bleeding and related complications, the apparent between-group difference was accounted for by less use of uterotonic agents in the tranexamic acid group than in the placebo group. There were no substantial between-group differences in the use of surgical or interventional radiologic procedures. Moreover, any benefit did not translate to fewer blood transfusions.
The risk of thromboembolic events (venous or arterial) was not higher in the tranexamic acid group than in the placebo group. In cases in which open-label tranexamic acid was used, our protocol allowed for only the intravenous administration of 1 g of tranexamic acid within the first 24 hours after the trial infusion was completed. Therefore, no participant should have received more than 2 g of tranexamic acid within a 24-hour period. The safety of higher doses requires further study. The incidence of postpartum infection was 3.2% in the tranexamic acid group and 2.5% in the placebo group.
Our findings are inconsistent with those of previous observational studies and systematic reviews suggesting that the use of prophylactic tranexamic acid during cesarean delivery reduces the use of blood transfusion.7,8,13,14 Many of these trials were small, single-center studies that were prone to biases, and none were adequately powered to evaluate the use of blood transfusion.15 A recent large, randomized, placebo-controlled trial (Tranexamic Acid for Preventing Postpartum Hemorrhage Following a Cesarean Delivery [TRAAP2]) also showed no significant difference in the use of blood transfusion with prophylactic administration of tranexamic acid at the time of cesarean delivery, but that trial was not powered to detect a difference in the incidence of transfusion.10 Another limitation of the TRAAP2 trial was that approximately 70% of the patients underwent cesarean delivery before the onset of labor, a situation that is associated with lower risk of bleeding and use of blood transfusion than cesarean delivery performed during labor.11,12 The authors found no between group difference in the use of uterotonic agents but did find that the calculated blood loss was significantly less in the tranexamic acid group than in the placebo group (mean difference, approximately 100 ml). The clinical significance of this difference is questionable.
Our trial was larger than previous randomized trials evaluating the role of prophylactic tranexamic acid at the time of cesarean delivery and was adequately powered to evaluate the effect on the use of blood transfusion. The incidence of the primary outcome was higher than that used in the sample-size estimation. Randomization was restricted to allow a maximum of 50% scheduled cesarean deliveries as compared with unscheduled deliveries; this approach was intended to prevent overrepresentation of scheduled cesarean deliveries, which are associated with a lower risk of blood transfusion. The pragmatic design of our trial, which allowed practitioners to manage postpartum hemorrhage (including transfusion thresholds) according to local protocols, increases the external validity of our findings. The trial cohort reflected ample representativeness of the population (Table S5).
This trial has some limitations. Because tranexamic acid was administered after umbilical-cord clamping, the benefit of earlier administration (before incision), if any, is unknown. Once tranexamic acid is administered intravenously, the concentration reaches a threshold of 10 μg per milliliter on average shortly after 3 minutes and persists above this level for at least 1 hour after delivery.16 Delays in the administration of tranexamic acid were infrequent; tranexamic acid or placebo was administered within 3 minutes after umbilical-cord clamping in 92% of the participants. The trial excluded patients who were at high risk for thromboembolic phenomena, such as those with a history of thromboembolic events and those with congenital or acquired thrombophilia. The safety of tranexamic acid in this population remains largely unknown and requires further study. Further study is also needed to confirm the finding of a higher incidence of postpartum infections with tranexamic acid, which was unexpected.
In this placebo-controlled trial, we found that prophylactic administration of tranexamic acid during cesarean delivery did not lead to a lower risk of maternal death or blood transfusion.
Supplementary Material
CLINICAL TRIAL REGISTRATION.
The Journal requires investigators to register their clinical trials in a public trials registry. The members of the International Committee of Medical Journal Editors (ICMJE) will consider most reports of clinical trials for publication only if the trials have been registered. Current information on requirements and appropriate registries is available at www.icmje.org/about-icmje/faqs/.
Acknowledgments
Supported by grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (UG1 HD087230, UG1 HD027869, UG1 HD027915, UG1 HD034208, UG1 HD040500, UG1 HD040485, UG1 HD053097, UG1 HD040544, UG1 HD040545, UG1 HD040560, UG1 HD040512, UG1 HD087192, and U24 HD036801).
We thank the patients who participated in this trial; the staff members who provided assistance at the clinical centers; Elizabeth A. Thom, Ph.D., for protocol development and trial management; and Uma M. Reddy, M.D., M.P.H., for protocol development.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
A data sharing statement provided by the authors is available with the full text of this article at NEJM.org.
Contributor Information
Luis D. Pacheco, University of Texas Medical Branch, Galveston, Texas.
Rebecca G. Clifton, George Washington University Biostatistics Center, Washington, DC.
George R. Saade, University of Texas Medical Branch, Galveston, Texas.
Steven J. Weiner, George Washington University Biostatistics Center, Washington, DC.
Samuel Parry, University of Pennsylvania, Philadelphia.
John M. Thorp, Jr, University of North Carolina at Chapel Hill, Chapel Hill.
Monica Longo, Eunice Kennedy Shriver National Institute of Child Health and Human Development, Bethesda, MD.
Ashley Salazar, University of Texas Medical Branch, Galveston, Texas.
Wendy Dalton, MetroHealth Medical Center, Case Western Reserve University, Cleveland, Ohio.
Alan T.N. Tita, University of Alabama at Birmingham, Birmingham.
Cynthia Gyamfi-Bannerman, Columbia University, New York.
Suneet P. Chauhan, University of Texas Health Science Center at Houston, Children’s Memorial Hermann Hospital, Houston, Texas.
Torri D. Metz, University of Utah Health Sciences Center, Salt Lake City.
Kara Rood, Ohio State University, Columbus, Ohio.
Dwight J. Rouse, Brown University, Providence, RI.
Jennifer L. Bailit, MetroHealth Medical Center, Case Western Reserve University, Cleveland, Ohio.
William A. Grobman, Northwestern University, Chicago.
Hyagriv N. Simhan, University of Pittsburgh, Pittsburgh.
George A. Macones, University of Texas at Austin, Austin, Texas.
REFERENCES
- 1.Say L, Chou D, Gemmill A, et al. Global causes of maternal death: a WHO systematic analysis. Lancet Glob Health 2014; 2(6): e323–e333. [DOI] [PubMed] [Google Scholar]
- 2.Davis NL, Smoots AN, Goodman DA. Pregnancy-related deaths: data from 14 U.S. maternal mortality review committees, 2008–2017. Atlanta: Centers for Disease Control and Prevention, 2019. (https://www.cdc.gov/reproductivehealth/maternal-mortality/erase-mm/mmr-data-brief_2019-h.pdf). [Google Scholar]
- 3.CRASH-2 trial collaborators. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): a randomised, placebo-controlled trial. Lancet 2010; 376: 23–32. [DOI] [PubMed] [Google Scholar]
- 4.CRASH-3 trial collaborators. Effects of tranexamic acid on death, disability, vascular occlusive events and other morbidities in patients with acute traumatic brain injury (CRASH-3): a randomised, placebo-controlled trial. Lancet 2019; 394: 1713–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.WOMAN Trial Collaborators. Effect of early tranexamic acid administration on mortality, hysterectomy, and other morbidities in women with post-partum haemorrhage (WOMAN): an international, randomised, double-blind, placebo-controlled trial. Lancet 2017; 389: 2105–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hellgren M Hemostasis during normal pregnancy and puerperium. Semin Thromb Hemost 2003; 29: 125–30. [DOI] [PubMed] [Google Scholar]
- 7.Ray I, Bhattacharya R, Chakraborty S, Bagchi C, Mukhopadhyay S. Role of intravenous tranexamic acid on caesarean blood loss: a prospective randomised study. J Obstet Gynaecol India 2016; 66: Suppl 1: 347–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lakshmi SD, Abraham R. Role of prophylactic tranexamic acid in reducing blood loss during elective cesarean section: a randomized controlled study. J Clin Diagn Res 2016; 10(12): QC17–QC21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sentilhes L, Winer N, Azria E, et al. Tranexamic acid for the prevention of blood loss after vaginal delivery. N Engl J Med 2018; 379: 731–42. [DOI] [PubMed] [Google Scholar]
- 10.Sentilhes L, Sénat MV, Le Lous M, et al. Tranexamic acid for the prevention of blood loss after cesarean delivery. N Engl J Med 2021; 384: 1623–34. [DOI] [PubMed] [Google Scholar]
- 11.Rouse DJ, MacPherson C, Landon M, et al. Blood transfusion and cesarean delivery. Obstet Gynecol 2006; 108: 891–7. [DOI] [PubMed] [Google Scholar]
- 12.Grobman WA, Bailit JL, Rice MM, et al. Can differences in obstetric outcomes be explained by differences in the care provided? The MFMU Network APEX study. Am J Obstet Gynecol 2014;2 11(2): 147.e1147.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Y, Liu S, He L. Prophylactic use of tranexamic acid reduces blood loss and transfusion requirements in patients undergoing cesarean section: a meta-analysis. J Obstet Gynaecol Res 2019; 45: 1562–75. [DOI] [PubMed] [Google Scholar]
- 14.Bellos I, Pergialiotis V. Tranexamic acid for the prevention of postpartum hemorrhage in women undergoing cesarean delivery: an updated meta-analysis. Am J Obstet Gynecol 2022; 226(4): 510–523. e22. [DOI] [PubMed] [Google Scholar]
- 15.Ker K, Shakur H, Roberts I. Does tranexamic acid prevent postpartum haemorrhage? A systematic review of randomised controlled trials. BJOG 2016; 123: 1745–52. [DOI] [PubMed] [Google Scholar]
- 16.Ahmadzia HK, Luban NLC, Li S, et al. Optimal use of intravenous tranexamic acid for hemorrhage prevention in pregnant women. Am J Obstet Gynecol 2021; 225(1): 85.e1–85.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


