Abstract
Background
Robot-assisted distal pancreatectomy (RDP) has been suggested to hold some benefits over laparoscopic distal pancreatectomy (LDP) but consensus and data on specific subgroups are lacking. This systematic review and meta-analysis reports the surgical and oncological outcome and costs between RDP and LDP including subgroups with intended spleen preservation and pancreatic ductal adenocarcinoma (PDAC).
Methods
Studies comparing RDP and LDP were included from PubMed, Cochrane Central Register, and Embase (inception-July 2022). Primary outcomes were conversion and unplanned splenectomy. Secondary outcomes were R0 resection, lymph node yield, major morbidity, operative time, intraoperative blood loss, in-hospital mortality, operative costs, total costs and hospital stay.
Results
Overall, 43 studies with 6757 patients were included, 2514 after RDP and 4243 after LDP. RDP was associated with a longer operative time (MD = 18.21, 95% CI 2.18–34.24), less blood loss (MD = 54.50, 95% CI − 84.49–24.50), and a lower conversion rate (OR = 0.44, 95% CI 0.36–0.55) compared to LDP. In spleen-preserving procedures, RDP was associated with more Kimura procedures (OR = 2.23, 95% CI 1.37–3.64) and a lower rate of unplanned splenectomies (OR = 0.32, 95% CI 0.24–0.42). In patients with PDAC, RDP was associated with a higher lymph node yield (MD = 3.95, 95% CI 1.67–6.23), but showed no difference in the rate of R0 resection (OR = 0.96, 95% CI 0.67–1.37). RDP was associated with higher total (MD = 3009.31, 95% CI 1776.37–4242.24) and operative costs (MD = 3390.40, 95% CI 1981.79–4799.00).
Conclusions
RDP was associated with a lower conversion rate, a higher spleen preservation rate and, in patients with PDAC, a higher lymph node yield and similar R0 resection rate, as compared to LDP. The potential benefits of RDP need to be weighed against the higher total and operative costs in future randomized trials.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00464-023-09894-y.
Keywords: Pancreas, Robot-assisted, Laparoscopy, Distal pancreatectomy, Meta-analysis, Subgroups
Distal pancreatectomy is the standard treatment for tumors in the body and tail of the pancreas. In recent years, robot-assisted distal pancreatectomy (RDP) and laparoscopic distal pancreatectomy (LDP) have increasingly been adopted. Many studies have suggested the safety, oncologic efficacy, and cost-effectiveness of both techniques as compared to the conventional open distal pancreatectomy (ODP) [1–3]. Two randomized trials have confirmed the superiority of LDP as compared to ODP in terms of time to functional recovery, hospital stay, and intraoperative blood loss [4, 5]. Therefore, the Miami Guidelines on minimally invasive pancreatic resection recommend the use of minimally invasive distal pancreatectomy (MIPD) over ODP for benign and low-grade malignant tumors [6]. For patients with left-sided pancreatic ductal adenocarcinoma (PDAC), guidelines state that in experienced hands minimally invasive distal pancreatectomy appears to be feasible, safe and oncologically equivalent to ODP, although prospective comparative studies are lacking [6].
More recently, interest has shifted towards the comparison between RDP and LDP. Some studies have suggested that RDP is associated with lower conversion rates, lower intraoperative blood loss, higher spleen preservation rates, and reduced hospital stay [7, 8]. On the other hand, RDP carries significantly higher costs which is considered a major drawback [9, 10]. Due to the absence of randomized trials, no superiority of any approach can be claimed. As RDP is associated with high costs, the choice for a robotic approach could include specific patient subgroups who benefit the most from such an approach. Most studies include patients operated for all indications and could, therefore, not advise the surgeon on the preferred approach in a certain patient. Therefore, the choice for RDP or LDP in an individual patient is currently based on the discretion of the operating surgeon, surgeons’ experience, and the availability of the robotic platform, and not on a high level of evidence. To enable future recommendation on the choice for RDP and LDP, more data on outcomes in specific patients subgroups who will benefit from a particular approach is needed.
This systematic review and meta-analysis aims to compare the surgical and oncological outcome of RDP and LDP in unselected patients, patients with intended spleen preservation and patients with PDAC by analyzing the largest number of published studies to date. In addition, a cost-analysis was performed to elaborate on the economic value of both approaches.
Methods
Study selection
A systematic review and meta-analysis was performed comparing RDP with LDP. An electronic search was performed in PubMed, Cochrane Central Register of Controlled Trials, and Embase, between inception and July 2022. Search terms included ‘distal pancreatectomy’, ‘minimally-invasive, ‘robot-assisted’ and ‘laparoscopic’ and synonyms. All identified publications were reviewed for inclusion by three reviewers (TVR, EAVB, and PZ) and inconsistencies were addressed by discussion and consensus among the reviewers. The screening process was done according to the PRISMA guidelines [11]. The identified articles were crosschecked on references. The study protocol was registered with PROSPERO (number CRD42022314724).
Eligibility criteria
Studies comparing RDP versus LDP for all indications and for subgroups were included. Studies with less than 10 patients were excluded. When multiple studies were reported from the same dataset, only the most recent publication was included in the analysis. Letters, editorials, case reports, expert opinions, systematic reviews, and meta-analyses were excluded.
Outcomes
Primary outcomes were conversion and unplanned splenectomy. Secondary outcomes were R0 resection, lymph node yield, major morbidity, operative time, intraoperative blood loss, in-hospital mortality, operative costs, total costs and hospital stay. Conversion was defined as any procedure that started as a robot-assisted of laparoscopic or procedure but required conversion to open surgery for a reason other than specimen extraction [12]. An unplanned splenectomy was defined as splenectomy in patients operated with the intention to preserve the spleen. Major morbidity was defined as a Clavien-Dindo grade 3a or higher complication [13]. Definition of clinically relevant pancreatic fistula followed the definitions of the International Study Group on Pancreatic Surgery (ISGPS), grade B/C[14] and the type of spleen-preserving procedure was classified according to the Kimura[15] or Warshaw[16] procedure.
Data extraction and management
A standardized data extraction form was used by the three independent reviewers (TVR, EAVB, and PZ). The following data were extracted from the included studies: first author, year of publication, study design, sample size of the groups, baseline characteristics, surgical details, all primary and secondary outcomes, postoperative care, operative costs and total costs.
Assessment of risk of bias in included studies
Quality of the studies (all non-RCTs) were assessed using the Newcastle-Ottawa scale [17]. The independent outcomes were assessed with the GRADE approach. Inconsistencies were assessed with the heterogeneity factor p and I2. Imprecision was calculated with the Optimal Information Size. Funnel plots were drawn for each outcome and assessed for symmetry to assess publication bias.
Statistical analysis
A meta-analysis was performed using R (The R Foundation for Statistical Computing, Vienna, Austria, version 4.1.3) with “metafor” and “varameta” package [18]. The results of continuous data (operation time, intraoperative blood loss, lymph node yield, operation cost and hospital stay) were calculated as the mean difference (MD) with 95% confidence intervals (CI’s). For studies reporting only median with range, median and standard deviation were calculated by the “varameta” package. Dichotomous outcome variables were reported as odds ratio’s (OR) with 95% CI’s. Heterogeneity was investigated with the chi-square and I2 test and interpreted as follows: 0% to 40% low, 30% to 60% moderate, 50% to 90% substantial, and 75% to 100% considerable. Imprecision of the included studies on the primary outcomes was determined by calculating Optimal Information Size [19]. A fixed effects model was used with a I2 index of lower than 50%. A random effects model was used with I2 > 50%. A potential publication bias for the primary outcomes was visually inspected by funnel plots and their symmetry was evaluated by Egger’s test [20]. The included studies are displayed in original national currency. Costs were recalculated to 2022 Dutch Euro by using purchasing power parities as provided by the OECD since this study is of Dutch origin. Sensitivity analysis were performed with leave-one-out meta-analysis by excluding each one study at a time to confirm the robustness of our findings [18].
Results
Overall, 872 studies were identified, of whom 241 duplicates were removed and 548 studies were excluded based on title and abstract. Of the 83 remaining studies, a full text publication could be obtained from 76 studies. Thereafter, 16 studies were excluded because no comparison was made between RDP versus LDP, and 17 further studies were excluded because the required primary outcomes were not reported. No studies were added after a reference crosscheck. Finally, 43 studies were included consisting of six prospective and 37 retrospective studies involving 6757 patients. Of these, 2514 patients underwent RDP and 4243 patients LDP [7, 8, 21–61]. A flowchart of the literature search is shown in Supplementary Fig. 1 and study characteristics in Table 1.
Table 1.
Characteristics of the included studies
| Author | Year | Study period | Study design | Country | n RDP/LDP | Age RDP/LDP (as reported) | BMI RDP/LDP (as reported) | Past surgical history RDP/LDP (%) |
|---|---|---|---|---|---|---|---|---|
| Alfieri S. [21] | 2019 | 2008–2016 | Retrospective | Italy | 96/85 | NA | NA | 48.9/41.1 |
| Baimas-George M. [22] | 2020 | 2009–2019 | Retrospective | USA | 33/42 | 68/71^ | 26.5/25.1^ | NA |
| Beniziri E. [23] | 2014 | 2004–2011 | Retrospective | USA | 11/23 | 50.1/52.3* | 25.6/26.5* | 54.4/43.5 |
| Butturini G. [24] | 2015 | 2011–2014 | Prospective | Italy | 22/21 | 54/55^ | 44.19/25.33^ | 68.2/61.9 |
| Chen P. [25] | 2022 | 2013–2019 | Retrospective | China | 54/95 | 50.06/51.74* | 24.23/24.23* | NA |
| Chen S. [26] | 2015 | 2005–2014 | Prospective PSM | China | 69/50 | 56.2/56.5* | 24.6/24.6* | 0/0 |
| Chopra A. [27] | 2021 | 2008–2019 | Retrospective | USA | 88/17 | NA | NA | 65.9/64.7 |
| Daouadi M. [28] | 2013 | 2008–2011 | Retrospective | USA | 30/94 | 59/59* | 27.9/29* | 73/51 |
| De Pastena M. [29] | 2020 | 2011–2017 | Retrospective PSM | Italy | 37/66 | 50/53^ | 24/24^ | NA |
| Di Franco G. [30] | 2022 | 2008–2020 | Retrospective PSM | Italy | 70/35 | Si 60.4 Xi 60.3/63.9^ | Si 26.2 Xi 26/26* | NA |
| Duran H. [31] | 2014 | 2008–2013 | Retrospective | Spain | 16/18 | 61/58.3* | NA | NA |
| Eckhardt S. [32] | 2016 | 2009–2015 | Retrospective | Germany | 12/29 | 48.5/59^ | 23/26.99^ | 0/0 |
| Esposito A. [33] | 2022 | 1999–2018 | Retrospective | Italy | 101/300 | NA | NA | 26.7/20.3 |
| Fisher A.V. [34] | 2019 | 2012–2014 | Retrospective | USA | 53/146 | 59/58^ | NA | NA |
| Goh B. K. P. [35] | 2017 | 2006–2015 | Retrospective | Singapore | 8/31 | 57/56^ | 27.6/23.9^ | 12.5/32.3 |
| Han J. H. [36] | 2018 | 2012–2018 | Retrospective | South Korea | 13/22 | 46.1/58.3* | 20.9/23.9* | 30.8/22.7 |
| Hong S. [37] | 2020 | 2015–2017 | Retrospective | South Korea | 46/182 | 51.2/60.2* | 24.9/24.6* | 32.6/28 |
| Ito M. [38] | 2014 | 2009–2013 | Retrospective | Japan | 4/10 | 52.7/68* | NA | NA |
| Jiang Y. [39] | 2020 | 2011–2018 | Retrospective | China | 63/103 | 44.5/48.8* | 22.8/22.6* | NA |
| Kamarajah S. [40] | 2022 | 2007–2018 | Retrospective | UK | 40/47 | 62/67^ | 28/28^ | NA |
| Kang C. [41] | 2010 | 2006–2010 | Retrospective | South Korea | 20/25 | 44.5/56.5* | 24.2/23.4* | NA |
| Kriger A.G. [42] | 2015 | 2009–2014 | Retrospective | Russia | 19/10 | 49.88/47.4* | NA | NA |
| Kwon J. [8] | 2021 | 2015–2020 | Retrospective PSM | South Korea | 104/208 | 50.62/51.23* | 24.05/24.06* | NA |
| Lai E. C. [43] | 2015 | 1999–2015 | Retrospective | China | 17/18 | 61.2/63.2* | 24.1/25.7* | NA |
| Lee S. Q. [44] | 2020 | 2006–2019 | Retrospective | Singapore | 27/75 | 64/61^ | 23.1/23.4^ | 18.5/30.7 |
| Lee S. Y. [45] | 2015 | 2000–2013 | Retrospective | USA | 37/131 | 58/58* | 28.7/28.2* | NA |
| Lin X.C. [46] | 2019 | 2016–2018 | Retrospective PSM | China | 41/41 | 45.2/47.4* | NA | NA |
| Liu R. [47] | 2017 | 2011–2015 | Retrospective PSM | China | 102/102 | 48.1/49.62* | NA | NA |
| Lof S. [7] | 2021 | 2011–2019 | Retrospective PSM | NL | 402/402 | 57/57* | 25.4/25.9* | 41/38.3 |
| Lyman W.B. [48] | 2019 | 2008–2017 | Retrospective | USA | 108/139 | 56.3/59.5* | 29.3/29* | NA |
| Magge D. [49] | 2018 | 2010–2016 | Retrospective | USA | 196/93 | 62.7/61.3* | 29.68/28.21* | NA |
| Marino M. [50] | 2020 | 2014–2017 | Retrospective PSM | Italy | 35/35 | 59.3/58.5^ | NA | 20/14.3 |
| Najafi N. [51] | 2020 | 2008–2015 | Retrospective | Germany | 24/32 | NA | NA | NA |
| Qu L. [52] | 2018 | 2011–2015 | Retrospective PSM | China | 35/35 | 58.1/57.8* | 24.46/24.08* | NA |
| Raoof M. [53] | 2018 | 2010–2013 | Retrospective | USA | 99/605 | NA | NA | NA |
| Rodriguez M. [54] | 2018 | 2012–2015 | Retrospective | France | 21/25 | 53/62.5^ | 25/27.3^ | 71.4/68 |
| Ryan C. E. [55] | 2015 | 2012–2014 | Prospective | USA | 18/16 | 68/58* | 28/25* | NA |
| Souche R. [56] | 2018 | 2011–2016 | Prospective | France | 15/23 | 57/66^ | 23/25^ | 13/21 |
| Vicente E. [57] | 2020 | 2011–2018 | Prospective | Spain | 31/28 | 59.9/61.5^ | 24.2/24.5^ | NA |
| Waters J. A. [58] | 2010 | 2008–2009 | Prospective | USA | 17/18 | 64/59" | NA | NA |
| Xourafas D. [59] | 2017 | Jan 2014–Dec 2014 | Retrospective | USA | 200/694 | 62/62^ | 28.8/28.4^ | NA |
| Yang S. J. [60] | 2020 | 2007–2018 | Retrospective | South Korea | 37/41 | 42.9/51.3* | 23.5/24.1* | NA |
| Zhang J. [61] | 2017 | 2010–2017 | Retrospective | China | 43/31 | 47.9/48.7* | 23.9/23.3* | NA |
*Mean, ^median, “unknown, PSM Propensity Score Matching, RDP robotic distal pancreatectomy, LDP laparoscopic distal pancreatectomy, BMI Body Mass Index, NA not applicable
Risk of bias assessment
The risk of bias is displayed in Supplementary Table 1. None of the included studies had a very high risk of bias (0 to 3 points) and the minimum risk of included studies was 7. Inconsistency was determined based on the heterogeneity factor p and I2 as shown in Table 2. For the primary outcomes, conversion and unplanned splenectomy, a low heterogeneity was found. For the secondary outcomes R0 resection, major morbidity and in-hospital mortality, a low heterogeneity was found, whereas for operative time, intraoperative blood loss, lymph node yield, operative costs, total costs and hospital stay a substantial heterogeneity was found. With an event rate between both groups of 36.7% for conversions and 54.4% for unplanned splenectomy, the optimal information size threshold (n = 2766) was met for the primary outcomes with an overall sample size of 6757 in this study.
Table 2.
Summary of findings with GRADE
| Outcome | No of studies | Risk of bias | Inconsistency | Indirectness | Imprecision | Quality GRADE | Statistical method | Effect estimate |
|---|---|---|---|---|---|---|---|---|
| Operative time in minutes | 40 | NSa | p = 0.00 Ib = 90.5% | NSb | NSc | Mod ⊕ ⊕ ⊕ ⊝ | Mean difference (REM, 95% CI) | 18.21 [2.18, 32.24] |
| Intraoperative blood loss in ml | 34 | NSa | p = 0.00 Ib = 91.9% | NSb | NSc | Mod ⊕ ⊕ ⊕ ⊝ | Mean difference (REM, 95% CI) | − 54.50 [− 84.49, − 24.50] |
| Conversion | 39 | NSa | p = 0.45 Ib = 1.1% | NS2 | NSc | High ⊕ ⊕ ⊕ ⊕ | Odds Ratio (M–H, FEM, 95% CI) | 0.44 [0.36, 0.55] |
| Unplanned splenectomy | 15 | NSa | p = 0.19 Ib = 23.7% | NSb | NSc | Mod ⊕ ⊕ ⊕ ⊝ | Odds Ratio (M–H, REM, 95% CI) | 0.32 [0.24, 0.42] |
| Kimura | 20 | NSa | p = 0.02 Ib = 53.0% | NSb | NSc | Mod ⊕ ⊕ ⊕ ⊝ | Odds Ratio (M–H, REM, 95% CI) | 2.23 [1.37, 3.64] |
| Blood transfusion | 22 | NSa | p = 0.68 Ib= 0.0% | NSb | NSc | Mod ⊕ ⊕ ⊕ ⊝ | Odds Ratio (M–H, FEM, 95% CI) | 0.93 [0.69, 1.25] |
| Major morbidity | 31 | NSa | p = 0.31 Ib = 9.7% | NSb | NSc | Mod ⊕ ⊕ ⊕ ⊝ | Odds Ratio (M–H, FEM, 95% CI) | 0.93 [0.76, 1.14] |
| POPF | 40 | NSa | p = 0.89 Ib = 0.0% | NSb | NSc | Mod ⊕ ⊕ ⊕ ⊝ | Odds Ratio (M–H, FEM, 95% CI) | 0.98 [0.85, 1.14] |
| Reoperation | 25 | NSa | p = 0.84 Ib = 0.0% | NS2 | NSc | Mod ⊕ ⊕ ⊕ ⊝ | Odds Ratio (M–H, FEM, 95% CI) | 0.94 [0.68, 1.31] |
| In-hospital mortality | 31 | NS1 | p = 1.00 Ib = 0.0% | NSb | NSc | Mod ⊕ ⊕ ⊕ ⊝ | Odds Ratio (M–H, FEM, 95% CI) | 1.40 [0.70, 2.82] |
| Hospital stay in days | 32 | NSa | p = 0.00 I2 = 71.3% | NSb | NSc | Low ⊕ ⊕ ⊝ ⊝ | Mean difference (REM, 95% CI) | − 0.45 [− 0.92, 0.01] |
| R0 resections in PDAC | 11 | NSa | p = 0.46 Ib = 0.0% | NSb | NSc | Mod ⊕ ⊕ ⊕ ⊝ | Odds Ratio (M–H, FEM, 95% CI) | 0.96 [0.67, 1.37] |
| Harvested lymph nodes | 10 | NSa | p = 0.00 Ib= 80.2% | NSb | NSc | Mod ⊕ ⊕ ⊕ ⊝ | Mean difference (REM, 95% CI) | 3.95 [1.67, 6.23] |
| Operative costs | 7 | NSa | p = 0.00 Ib = 99.5% | NSb | NSc | Mod ⊕ ⊕ ⊕ ⊝ | Mean difference (REM, 95% CI) | 3390.40 [1981.79, 4799.00] |
| Total costs | 9 | NSa | p = 0.00 Ib = 95.0% | NSb | NSc | Mod ⊕ ⊕ ⊕ ⊝ | Mean difference (REM, 95% CI) | 3009.31 [1776.37, 4242.25] |
POPF postoperative pancreatic fistula; NS Not serious; MD Mean difference; REM random effects model; FEM fixed effects model
aAccording to the assessment of risk of bias, the included studies all have a low risk of bias (supplementary Table 1)
bThe results of these variables for the included studies have no serious effect on the indirectness since the studies relate well to the aim of current study
cTo determine if imprecision was an influence on the quality of the studies, the Optimal Information Size was calculated using the GRADE approach for the outcome of major morbidity. With an event rate between groups of 36.7% for conversions, 54,4% for unplanned splenectomy and 82.3% for Kimura, the optimal information size threshold was met for the primary outcomes since this implicates that a sample size of minimally 2766 is required
Publication bias
Funnel plots of publications reporting on the outcomes of interest were symmetrical and all statistically verified (Egger’s test; conversion: p = 0.35, unplanned splenectomy: p = 0.14, major morbidity: p = 0.14, in-hospital; mortality: p = 0.71, CR-POPF: p = 0.35, reoperation: p = 0.47, intraoperative blood transfusion: p = 0.19, intraoperative blood loss: p = 0.71, operative time: p = 0.87, hospital stay: p = 0.05, R0 resection: p = 0.32, lymph node yield: p = 0.09, operation costs: p = 0.75, total costs: p = 0.61). The funnel plots for the primary outcomes are shown in Supplementary Fig. 2a (conversion) and 2b (unplanned splenectomy).
Total cohort
Preoperative characteristics
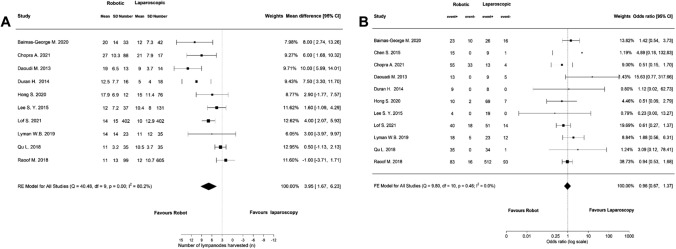
The meta-analyses of preoperative patient and tumor characteristics are shown in Supplementary Fig. 3a–d. The RDP cohort included younger patients (MD − 1.66 years, 95% CI: − 2.42 to -0.89) with smaller tumors (MD − 2.75 mm, 95% CI: − 4.52 to -0.98) and more patients with previous abdominal surgery (OR: 1.22, 95% CI: 1.01 to 1.48). BMI did not differ between the RDP and LDP group surgery (MD − 0.10 kg/m2, 95% CI: − 0.37 to 0.17).
Perioperative outcome
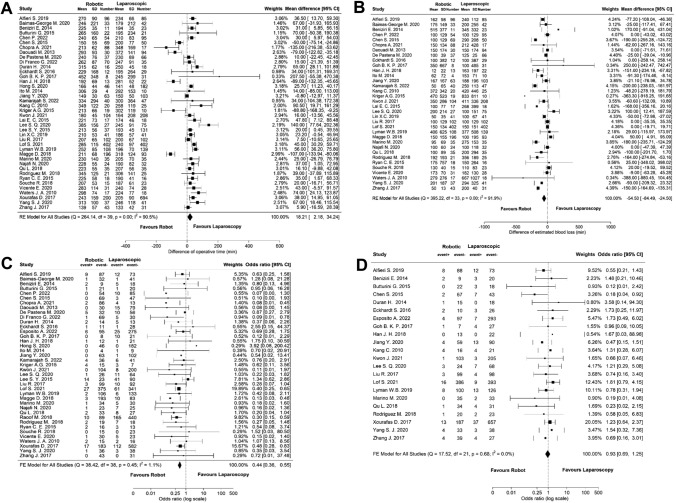
The forest plots of perioperative outcomes are displayed in Fig. 1a–d. RDP was associated with a significantly longer operative time (MD 18.21 min, 95% CI: 2.18 to 34.24) but less intraoperative blood loss (MD − 54.50 mL, 95% CI: − 84.49 to − 24,50) compared to LDP with no significant difference between both groups regarding the rate of intraoperative blood transfusion (OR 0.93, 95% CI: 0.65 to 1.25). The conversion rate was significantly lower in RDP (OR 0.44, 95% CI: 0.36 to 0.55).
Fig. 1.
Meta-analyses of the perioperative outcomes of the total cohort; A operative time, B intraoperative blood loss, C conversion, D intraoperative blood transfusion
Postoperative outcome
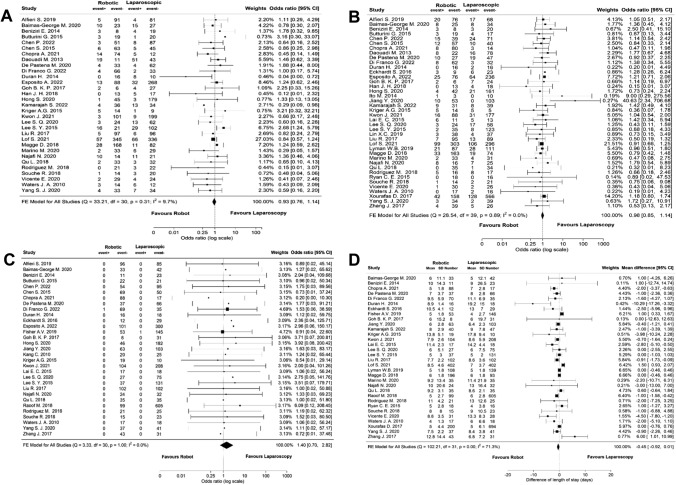
No significant differences were observed between RDP and LDP regarding all postoperative outcomes. The meta-analyses of major morbidity, POPF, in-hospital mortality and hospital stay are shown in Figs. 2a–d. The shorter hospital stay in the RDP group was not statistically significant (MD − 0.45 days, 95% CI: − 0.92 to 0.01).
Fig. 2.
Meta-analyses of the postoperative outcomes of the total cohort; A major morbidity, B postoperative pancreatic fistula, C in-hospital mortality, D hospital stay
Subgroup analysis splenic preservation
Of the 43 included studies, 20 reported outcomes specifically for spleen-preserving distal pancreatectomy. Meta-analysis of these studies revealed that significantly more Kimura (i.e. splenic vessel preserving) procedures were performed in the RDP group (Fig. 3a, OR 2.23, 95% CI: 1.37 to 3.64). In total, 15 studies assessed the rate of unplanned splenectomy and meta-analysis showed a significantly lower rate of unplanned splenectomies in the RDP group (Fig. 3b, OR 0.32, 95% CI: 0.24 to 0.42). The rate of conversion in these patients did not differ between both groups (Fig. 3c, OR 0.53, 95% CI: 0.26 to 1.09). Operative time was reported in 10 studies, showing no significant difference between RDP and LDP (Fig. 3d, MD 21.31, 95% CI: -1.25 to 43.86).
Fig. 3.
Meta-analyses of the outcomes in patients with intended spleen preservation; A Kimura technique, B unplanned splenectomy, C conversion, D operative time
Subgroup analysis PDAC
Of the 43 included studies, 11 reported on oncological outcomes specifically in patients with PDAC. Meta-analyses of these studies revealed a significant higher lymph node yield in the RDP group compared to LDP (Fig. 4a, MD 3.95 95% CI: 1.67 to 6.23), but no difference in the rate of R0 resection (Fig. 4b, OR 0.96, 95% CI: 0.67 to 1.37). Five studies reported on overall survival and three studies on disease-free survival but the data were insufficient to perform a meta-analysis.
Fig. 4.
Meta-analyses of the oncological outcomes in patients with PDAC; A lymph node yield, B R0 resection
Cost analysis
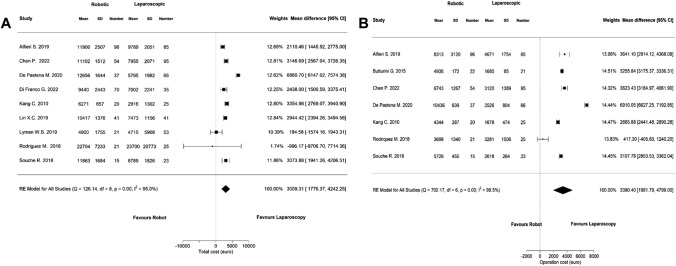
Nine studies reported on the total costs of RDP and LDP and meta-analysis of these studies showed that RDP was significantly more expensive than LDP (Fig. 5a, MD 3009.31, 95% CI: 1776.37 to 4242.24). Operative costs were reported in seven studies and were also significantly higher in RDP (Fig. 5b, MD 3390.40, 95% CI: 1981.79 to 4799.00).
Fig. 5.
Meta-analyses of the costs; A total costs, B operative costs
Leave-one-out analysis
In the leave-one-out analyses, focusing only on the significant differences identified, only previous abdominal surgery showed sensitivity and was no longer significant different between RDP and LDP when leaving out one of the following studies: Alfieri S. 2019, p = 0.066 [21], Daouadi M. 2019, p = 0.094 [28], Esposito A. 2022, p = 0.088 [33].
Discussion
In this largest systematic review and meta-analysis to date, including specific subgroups, RDP was associated with a lower conversion rate and, in patients with intended spleen preservation, with less unplanned splenectomies and a higher rate of splenic vessels preserving Kimura procedures. RDP was also associated with less intraoperative blood loss as compared to LDP at the cost of longer operative time. In patients with PDAC, RDP showed a higher lymph node yield with comparable R0 rates, as compared to LDP. As expected, RDP was associated with higher costs, as compared to LDP, approximating EUR 3000 per procedure.
In recent years, along with the increasing implementation of minimally invasive distal pancreatectomy, several meta-analyses comparing RDP and LDP have been published [9, 10, 62–70]. However, most of them are obsolete today, reported on half of the available evidence to date or included all indications without distinguishing subgroups. The current systematic review provides a complete and up-to-date analysis, including the most recent studies and around double the number of patients compared to the two most recent meta-analyses on RDP versus LDP for all indications [62, 64], thus contributing to the highest body of evidence in the absence of randomized trials. Moreover, analyses were performed in specific subgroups to demonstrate potential benefits of a particular approach.
Previous meta-analyses also described lower conversion rates of RDP and most of them found longer operative times in RDP [62–64]. Although the outcomes on operative time varied in previous literature, the current study confirmed the prolonged operative time of RDP. This could, at least partially, be explained by the additional time required for preparation and docking of the robot. With respect to other perioperative factors, RDP was associated with less intraoperative blood loss in this study, a finding which has found significant in only two previous meta-analyses potentially because of a type II error [65, 70]. It is assumed that the robotic platform allows for better prevention and control of bleeding due to the greater instrument dexterity, 3D high-definition visualization, and tremor filtration.
In the subgroup group analysis of patients with an intended spleen preservation, RDP was associated with a higher rate of Kimura procedures. A recent meta-analysis of only SPDP studies reported a rate of 81.1% Kimura procedures in the RDP group versus 54.5% in the LDP group, but did not assess its significance in a forest plot [70]. The present study corroborates these findings by showing significance in a forest plot. In general, the Kimura technique is regarded as the preferred procedure in patients planned for a spleen-preserving procedure when there is no tumor proximity or involvement to the splenic vessels [71], which is confirmed by a survey from 2018 that concluded that 82.5% of the surgeons attempt a Kimura procedure if feasible [72]. However, this approach is considered technically challenging due to the difficulty of separating the splenic vessels and dividing their branches from the pancreas. The technical features of the robot may be advantageous in this regard, which could be a reasonable explanation for the higher proportion of Kimura procedures in the RDP group. Interestingly, in SPDP, RDP was no longer associated with a longer operative time as compared to LDP. This may indicate that in such technically complex procedures, RDP loses its relative disadvantage of a longer operative time. In addition, in the subgroup analysis of SPDP, a lower rate of unplanned splenectomies was observed in the RDP cohort compared to the LDP cohort, what aligns with the often described higher spleen preservation rates of RDP in previous meta-analyses [62, 64, 70].
Oncological results of the subgroup of patients with PDAC revealed a higher lymph node yield in RDP with similar R0 resection rates compared to LDP based on 11 included studies. Studies comparing RDP with LDP for PDAC are scarce, but a recently published meta-analysis included six studies that reported outcomes for PDAC [68]. The results of that study showed opposite results to the present study, as RDP was associated with a higher R0 resection rate but a similar lymph node yield compared to LDP. However, only six studies were included for the R0 resection and five studies for the lymph node yield analyses. Contrarily, the current study included almost double that number of studies, with 11 studies on R0 resection rates and 10 studies on lymph node yield.
The results of this study should be interpreted in light of some limitations. First, the current study analyzed several patient and tumor characteristics and found that patients in the RDP group were significantly younger and had smaller tumors. This might indicate that in the first phase of the implementation of RDP more easily operable patients and tumors were selected for a robot-assisted approach. Despite this being an interesting finding, it is also a limitation of the study as it may have contributed to some outcomes, such as the lower blood loss. Second, all of the included studies were observational cohort studies and no randomized controlled trials are yet available. Additional selection bias, other than the identified differences, is therefore likely present, even though studies did attempt to minimize the bias by, for example, correct for confounding through matching of the cohorts. Third, data on 1- and 3-year survival were lacking in the majority of the studies so no firm conclusions can be drawn on survival differences between RDP and LDP. This important oncological outcome has still to be proven by future prospective data. The main strength of this meta-analysis is that it included the largest number of studies and patients to date (43 studies, 6757 patients) as compared to the largest in current literature (21 studies, 3463 patients) [64]. With additional analyses on subgroups and costs, while adopting a robust and more comprehensive method to minimize all potential forms of bias, the current study provides the highest level of evidence on the comparison between RDP and LDP.
Conclusions
This systematic review and meta-analysis found RDP associated with a higher rate of spleen preservation, a lower conversion rate, and similar postoperative outcomes as compared to LDP. RDP seems to be an oncological safe alternative to LDP given the equal R0 resection rate and higher lymph node yield. Potential disadvantages of RDP are the higher costs and longer operative time. Based on these results, and acknowledging the potential impact of bias in patients selection, RDP may be preferred over LDP in patients with benign lesions planned for a complex or Kimura intended spleen-preserving procedure. However, future randomized controlled trials are needed to confirm these findings and weigh the potential benefits and downsides of RDP with the associated costs.
Supplementary Information
Below is the link to the electronic supplementary material.
Funding
This research received no external funding.
Declarations
Disclosures
Drs. Tess van Ramshorst, Drs. Eduard van Bodegraven, Mr. Pietro Zampedri, Drs. Meidai Kasai, Prof. Marc Besselink and Prof. Abu Hilal have no conflicts of interest to disclose.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Tess M.E. van Ramshorst and Eduard A. van Bodegraven shared first authorships
Marc G. Besselink and Mohammad Abu Hilal shared senior authorships
Contributor Information
Tess M. E. van Ramshorst, Email: t.vanramshorst@amsterdamumc.nl
Mohammad Abu Hilal, Email: abuhilal9@gmail.com.
References
- 1.Lyu Y, Cheng Y, Wang B, Zhao S, Chen L. Comparison of 3 minimally invasive methods versus open distal pancreatectomy: a systematic review and network meta-analysis. Surg Laparosc Endosc Percutan Tech. 2020;31:104–112. doi: 10.1097/SLE.0000000000000846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abu Hilal M, Richardson JR, de Rooij T, Dimovska E, Al-Saati H, Besselink MG. Laparoscopic radical ‘no-touch’ left pancreatosplenectomy for pancreatic ductal adenocarcinoma: technique and results. Surg Endosc. 2016;30:3830–3838. doi: 10.1007/s00464-015-4685-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Hilst J, de Rooij T, Klompmaker S, Rawashdeh M, Aleotti F, Al-Sarireh B, Alseidi A, Ateeb Z, Balzano G, Berrevoet F, Bjornsson B, Boggi U, Busch OR, Butturini G, Casadei R, Del Chiaro M, Chikhladze S, Cipriani F, van Dam R, Damoli I, van Dieren S, Dokmak S, Edwin B, van Eijck C, Fabre JM, Falconi M, Farges O, Fernandez-Cruz L, Forgione A, Frigerio I, Fuks D, Gavazzi F, Gayet B, Giardino A, Groot Koerkamp B, Hackert T, Hassenpflug M, Kabir I, Keck T, Khatkov I, Kusar M, Lombardo C, Marchegiani G, Marshall R, Menon KV, Montorsi M, Orville M, de Pastena M, Pietrabissa A, Poves I, Primrose J, Pugliese R, Ricci C, Roberts K, Rosok B, Sahakyan MA, Sanchez-Cabus S, Sandstrom P, Scovel L, Solaini L, Soonawalla Z, Souche FR, Sutcliffe RP, Tiberio GA, Tomazic A, Troisi R, Wellner U, White S, Wittel UA, Zerbi A, Bassi C, Besselink MG, Abu Hilal M, European Consortium on Minimally Invasive Pancreatic S Minimally invasive versus open distal pancreatectomy for ductal adenocarcinoma (DIPLOMA): a pan-european propensity score matched study. Ann Surg. 2019;269:10–17. doi: 10.1097/SLA.0000000000002561. [DOI] [PubMed] [Google Scholar]
- 4.Bjornsson B, Larsson AL, Hjalmarsson C, Gasslander T, Sandstrom P. Comparison of the duration of hospital stay after laparoscopic or open distal pancreatectomy: randomized controlled trial. Br J Surg. 2020;107:1281–1288. doi: 10.1002/bjs.11554. [DOI] [PubMed] [Google Scholar]
- 5.de Rooij T, van Hilst J, van Santvoort H, Boerma D, van den Boezem P, Daams F, van Dam R, Dejong C, van Duyn E, Dijkgraaf M, van Eijck C, Festen S, Gerhards M, Groot Koerkamp B, de Hingh I, Kazemier G, Klaase J, de Kleine R, van Laarhoven C, Luyer M, Patijn G, Steenvoorde P, Suker M, Abu Hilal M, Busch O, Besselink M, Dutch Pancreatic Cancer G. Minimally invasive versus open distal pancreatectomy (LEOPARD): a multicenter patient-blinded randomized controlled trial. Ann Surg. 2019;269:2–9. doi: 10.1097/SLA.0000000000002979. [DOI] [PubMed] [Google Scholar]
- 6.Asbun HJ, Moekotte AL, Vissers FL, Kunzler F, Cipriani F, Alseidi A, D'Angelica MI, Balduzzi A, Bassi C, Bjornsson B, Boggi U, Callery MP, Del Chiaro M, Coimbra FJ, Conrad C, Cook A, Coppola A, Dervenis C, Dokmak S, Edil BH, Edwin B, Giulianotti PC, Han HS, Hansen PD, van der Heijde N, van Hilst J, Hester CA, Hogg ME, Jarufe N, Jeyarajah DR, Keck T, Kim SC, Khatkov IE, Kokudo N, Kooby DA, Korrel M, de Leon FJ, Lluis N, Lof S, Machado MA, Demartines N, Martinie JB, Merchant NB, Molenaar IQ, Moravek C, Mou YP, Nakamura M, Nealon WH, Palanivelu C, Pessaux P, Pitt HA, Polanco PM, Primrose JN, Rawashdeh A, Sanford DE, Senthilnathan P, Shrikhande SV, Stauffer JA, Takaori K, Talamonti MS, Tang CN, Vollmer CM, Wakabayashi G, Walsh RM, Wang SE, Zinner MJ, Wolfgang CL, Zureikat AH, Zwart MJ, Conlon KC, Kendrick ML, Zeh HJ, Hilal MA, Besselink MG, International Study Group on Minimally Invasive Pancreas S The miami international evidence-based guidelines on minimally invasive pancreas resection. Ann Surg. 2020;271:1–14. doi: 10.1097/SLA.0000000000003590. [DOI] [PubMed] [Google Scholar]
- 7.Lof S, van der Heijde N, Abuawwad M, Al-Sarireh B, Boggi U, Butturini G, Capretti G, Coratti A, Casadei R, D'Hondt M, Esposito A, Ferrari G, Fusai G, Giardino A, Groot Koerkamp B, Hackert T, Kamarajah S, Kauffmann EF, Keck T, Marudanayagam R, Nickel F, Manzoni A, Pessaux P, Pietrabissa A, Rosso E, Salvia R, Soonawalla Z, White S, Zerbi A, Besselink MG, Abu Hilal M. Robotic versus laparoscopic distal pancreatectomy: multicentre analysis. Br J Surg. 2021;108:188–195. doi: 10.1093/bjs/znaa039. [DOI] [PubMed] [Google Scholar]
- 8.Kwon J, Lee JH, Park SY, Park Y, Lee W, Song KB, Hwang DW, Kim SC. A comparison of robotic versus laparoscopic distal pancreatectomy: propensity score matching analysis. Int J Med Robot Computer Assist Surg. 2021;18(2):e2347. doi: 10.1002/rcs.2347. [DOI] [PubMed] [Google Scholar]
- 9.Di Martino M, Caruso R, D'Ovidio A, Nunez-Alfonsel J, Burdio Pinilla F, Quijano Collazo Y, Vicente E, Ielpo B. Robotic versus laparoscopic distal pancreatectomies: a systematic review and meta-analysis on costs and perioperative outcome. Int J Med Robot. 2021;17:e2295. doi: 10.1002/rcs.2295. [DOI] [PubMed] [Google Scholar]
- 10.Xu SB, Jia CK, Wang JR, Zhang RC, Mou YP. Do patients benefit more from robot assisted approach than conventional laparoscopic distal pancreatectomy? A meta-analysis of perioperative and economic outcomes. J Formos Med Assoc. 2019;118:268–278. doi: 10.1016/j.jfma.2018.05.003. [DOI] [PubMed] [Google Scholar]
- 11.Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA, Group P-P Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4(1):1–9. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Montagnini AL, Rosok BI, Asbun HJ, Barkun J, Besselink MG, Boggi U, Conlon KC, Fingerhut A, Han HS, Hansen PD, Hogg ME, Kendrick ML, Palanivelu C, Shrikhande SV, Wakabayashi G, Zeh H, Vollmer CM, Kooby DA. Standardizing terminology for minimally invasive pancreatic resection. HPB (Oxford) 2017;19:182–189. doi: 10.1016/j.hpb.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 13.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bassi C, Marchegiani G, Dervenis C, Sarr M, Abu Hilal M, Adham M, Allen P, Andersson R, Asbun HJ, Besselink MG, Conlon K, Del Chiaro M, Falconi M, Fernandez-Cruz L, Fernandez-Del Castillo C, Fingerhut A, Friess H, Gouma DJ, Hackert T, Izbicki J, Lillemoe KD, Neoptolemos JP, Olah A, Schulick R, Shrikhande SV, Takada T, Takaori K, Traverso W, Vollmer CR, Wolfgang CL, Yeo CJ, Salvia R, Buchler M, International Study Group on Pancreatic S The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 years after. Surgery. 2017;161:584–591. doi: 10.1016/j.surg.2016.11.014. [DOI] [PubMed] [Google Scholar]
- 15.Kimura W, Inoue T, Futakawa N, Shinkai H, Han I, Muto T. Spleen-preserving distal pancreatectomy with conservation of the splenic artery and vein. Surgery. 1996;120:885–890. doi: 10.1016/S0039-6060(96)80099-7. [DOI] [PubMed] [Google Scholar]
- 16.Warshaw AL. Conservation of the spleen with distal pancreatectomy. Arch Surg. 1988;123:550–553. doi: 10.1001/archsurg.1988.01400290032004. [DOI] [PubMed] [Google Scholar]
- 17.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 18.Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010;36:1–48. doi: 10.18637/jss.v036.i03. [DOI] [Google Scholar]
- 19.Garcia-Alamino JM, Bankhead C, Heneghan C, Pidduck N, Perera R. Impact of heterogeneity and effect size on the estimation of the optimal information size: analysis of recently published meta-analyses. BMJ Open. 2017;7:e015888. doi: 10.1136/bmjopen-2017-015888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alfieri S, Butturini G, Boggi U, Pietrabissa A, Morelli L, Vistoli F, Damoli I, Peri A, Fiorillo C, Pugliese L, Ramera M, De Lio N, Di Franco G, Esposito A, Landoni L, Rosa F, Menghi R, Doglietto GB, Quero G. Short-term and long-term outcomes after robot-assisted versus laparoscopic distal pancreatectomy for pancreatic neuroendocrine tumors (pNETs): a multicenter comparative study. Langenbecks Arch Surg. 2019;404:459–468. doi: 10.1007/s00423-019-01786-x. [DOI] [PubMed] [Google Scholar]
- 22.Baimas-George M, Watson M, Salibi P, Tschuor C, Murphy KJ, Iannitti D, Baker E, Ocuin L, Vrochides D, Martinie JB. Oncologic outcomes of robotic left pancreatectomy for pancreatic adenocarcinoma a single-center comparison to laparoscopic resection. Am surg. 2020;87(1):45–49. doi: 10.1177/0003134820949524. [DOI] [PubMed] [Google Scholar]
- 23.Benizri EI, Germain A, Ayav A, Bernard JL, Zarnegar R, Benchimol D, Bresler L, Brunaud L. Short-term perioperative outcomes after robot-assisted and laparoscopic distal pancreatectomy. J Robot Surg. 2014;8:125–132. doi: 10.1007/s11701-013-0438-8. [DOI] [PubMed] [Google Scholar]
- 24.Butturini G, Damoli I, Crepaz L, Malleo G, Marchegiani G, Daskalaki D, Esposito A, Cingarlini S, Salvia R, Bassi C. A prospective non-randomised single-center study comparing laparoscopic and robotic distal pancreatectomy. Surg Endosc. 2015;29:3163–3170. doi: 10.1007/s00464-014-4043-3. [DOI] [PubMed] [Google Scholar]
- 25.Chen P, Zhou B, Wang T, Hu X, Ye Y, Guo W. Comparative efficacy of robot-assisted and laparoscopic distal pancreatectomy: a single-center comparative study. J Healthc Eng. 2022;2022:7302222. doi: 10.1155/2022/7302222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen S, Zhan Q, Chen JZ, Jin JB, Deng XX, Chen H, Shen BY, Peng CH, Li HW. Robotic approach improves spleen-preserving rate and shortens postoperative hospital stay of laparoscopic distal pancreatectomy: a matched cohort study. Surg Endosc. 2015;29:3507–3518. doi: 10.1007/s00464-015-4101-5. [DOI] [PubMed] [Google Scholar]
- 27.Chopra A, Nassour I, Zureikat A, Paniccia A. Perioperative and oncologic outcomes of open, laparoscopic, and robotic distal pancreatectomy for pancreatic adenocarcinoma. Updat Surg. 2021;73:947–953. doi: 10.1007/s13304-020-00927-y. [DOI] [PubMed] [Google Scholar]
- 28.Daouadi M, Zureikat AH, Zenati MS, Choudry H, Tsung A, Bartlett DL, Hughes SJ, Lee KK, Moser AJ, Zeh HJ. Robot-assisted minimally invasive distal pancreatectomy is superior to the laparoscopic technique. Ann Surg. 2013;257:128–132. doi: 10.1097/SLA.0b013e31825fff08. [DOI] [PubMed] [Google Scholar]
- 29.De Pastena M, Esposito A, Paiella S, Surci N, Montagnini G, Marchegiani G, Malleo G, Secchettin E, Casetti L, Ricci C, Landoni L, Bovo C, Bassi C, Salvia R. Cost-effectiveness and quality of life analysis of laparoscopic and robotic distal pancreatectomy: a propensity score-matched study. Surg Endosc. 2021;35:1420–1428. doi: 10.1007/s00464-020-07528-1. [DOI] [PubMed] [Google Scholar]
- 30.Di Franco G, Peri A, Lorenzoni V, Palmeri M, Furbetta N, Guadagni S, Gianardi D, Bianchini M, Pollina LE, Melfi F, Mamone D, Milli C, Di Candio G, Turchetti G, Pietrabissa A, Morelli L. Minimally invasive distal pancreatectomy: a case-matched cost-analysis between robot-assisted surgery and direct manual laparoscopy. Surg Endosc. 2022;36:651–662. doi: 10.1007/s00464-021-08332-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Duran H, Ielpo B, Caruso R, Ferri V, Quijano Y, Diaz E, Fabra I, Oliva C, Olivares S, Vicente E. Does robotic distal pancreatectomy surgery offer similar results as laparoscopic and open approach? A comparative study from a single medical center. Int J Med Robot Computer Assist Surg. 2014;10:280–285. doi: 10.1002/rcs.1569. [DOI] [PubMed] [Google Scholar]
- 32.Eckhardt S, Schicker C, Maurer E, Fendrich V, Bartsch DK. Robotic-assisted approach improves vessel preservation in spleen-preserving distal pancreatectomy. Dig Surg. 2016;33:406–413. doi: 10.1159/000444269. [DOI] [PubMed] [Google Scholar]
- 33.Esposito A, Ramera M, Casetti L, De Pastena M, Fontana M, Frigerio I, Giardino A, Girelli R, Landoni L, Malleo G, Marchegiani G, Paiella S, Pea A, Regi P, Scopelliti F, Tuveri M, Bassi C, Salvia R, Butturini G. Surg Endosc. 2022;36:1–13. [Google Scholar]
- 34.Fisher AV, Fernandes-Taylor S, Schumacher JR, Havlena JA, Wang X, Lawson EH, Ronnekleiv-Kelly SM, Winslow ER, Weber SM, Abbott DE. Analysis of 90-day cost for open versus minimally invasive distal pancreatectomy. HPB. 2019;21:60–66. doi: 10.1016/j.hpb.2018.07.003. [DOI] [PubMed] [Google Scholar]
- 35.Goh BK, Chan CY, Soh HL, Lee SY, Cheow PC, Chow PK, Ooi LL, Chung AY. A comparison between robotic-assisted laparoscopic distal pancreatectomy versus laparoscopic distal pancreatectomy. Int J Med Robot. 2017;13:e1733. doi: 10.1002/rcs.1733. [DOI] [PubMed] [Google Scholar]
- 36.Han HJ, Kang CM. Reduced port minimally invasive distal pancreatectomy: single-port laparoscopic versus robotic single-site plus one-port distal pancreatectomy. Surg Endosc. 2019;33:1091–1099. doi: 10.1007/s00464-018-6361-3. [DOI] [PubMed] [Google Scholar]
- 37.Hong S, Ahmad M, Song KB, Ma CH, Kim SC, Lee YJ, Hwang DW, Lee JH, Shin SH, Kwon J, Hwang S, Park G, Park Y, Lee SJ, Park KM. Does robotic system have advantages over laparoscopic system for distal pancreatectomy? Surg Endosc Other Interv Tech. 2018;32:S349. [Google Scholar]
- 38.Ito M, Asano Y, Shimizu T, Uyama I, Horiguchi A. Comparison of standard laparoscopic distal pancreatectomy with minimally invasive distal pancreatectomy using the da Vinci S system. Hepatogastroenterology. 2014;61:493–496. [PubMed] [Google Scholar]
- 39.Jiang Y, Zheng K, Zhang S, Shao Z, Cheng P, Zhang Y, Jin G, He T. Robot-assisted distal pancreatectomy improves spleen preservation rate versus laparoscopic distal pancreatectomy for benign and low-grade malignant lesions of the pancreas. Translational Cancer Res. 2020;9:5166–5172. doi: 10.21037/tcr-19-2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kamarajah S, Sutandi N, Sen G, Hammond J, Manas D, French J, White S. Comparative analysis of open, laparoscopic and robotic distal pancreatic resection: the United Kingdom’s first single-centre experience. J Minim Access Surg. 2022;18:77–83. doi: 10.4103/jmas.JMAS_163_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kang CM, Kim DH, Lee WJ, Chi HS. Conventional laparoscopic and robot-assisted spleen-preserving pancreatectomy: does da Vinci have clinical advantages? Surg Endosc. 2011;25:2004–2009. doi: 10.1007/s00464-010-1504-1. [DOI] [PubMed] [Google Scholar]
- 42.Kriger AG, Berelavichus SV, Smirnov AV, Gorin DS, Akhtanin EA. Comparative results of open robot-assisted and laparoscopic distal pancreatic resection. Khirurgiia. 2015;1:23–29. doi: 10.17116/hirurgia2015123-29. [DOI] [PubMed] [Google Scholar]
- 43.Lai EC, Tang CN. Robotic distal pancreatectomy versus conventional laparoscopic distal pancreatectomy: a comparative study for short-term outcomes. Front med. 2015;9:356–360. doi: 10.1007/s11684-015-0404-0. [DOI] [PubMed] [Google Scholar]
- 44.Lee SQ, Kabir T, Koh YX, Teo JY, Lee SY, Kam JH, Cheow PC, Jeyaraj PR, Chow PKH, Ooi LL, Chung AYF, Chan CY, Goh BKP. A single institution experience with robotic and laparoscopic distal pancreatectomies. Ann Hepatobiliary Pancreat Surg. 2020;24:283–291. doi: 10.14701/ahbps.2020.24.3.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee SY, Allen PJ, Sadot E, D'Angelica MI, Dematteo RP, Fong Y, Jarnagin WR, Kingham TP. Distal pancreatectomy: a single institution’s experience in open, laparoscopic, and robotic approaches. J Am Coll Surg. 2015;220:18–27. doi: 10.1016/j.jamcollsurg.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 46.Lin XC, Huang HG, Chen YC, Lu FC, Lin RG, Yang YY, Wang CF, Fang HZ. Robotic versus laparoscopic distal pancreatectomy: a retrospective single-center study. Zhonghua Wai Ke Za Zhi. 2019;57:102–107. doi: 10.3760/cma.j.issn.0529-5815.2019.02.006. [DOI] [PubMed] [Google Scholar]
- 47.Liu R, Liu Q, Zhao ZM, Tan XL, Gao YX, Zhao GD. Robotic versus laparoscopic distal pancreatectomy: a propensity score-matched study. J Surg Oncol. 2017;116:461–469. doi: 10.1002/jso.24676. [DOI] [PubMed] [Google Scholar]
- 48.Lyman WB, Passeri M, Sastry A, Cochran A, Iannitti DA, Vrochides D, Baker EH, Martinie JB. Robotic-assisted versus laparoscopic left pancreatectomy at a high-volume, minimally invasive center. Surg Endosc. 2019;33:2991–3000. doi: 10.1007/s00464-018-6565-6. [DOI] [PubMed] [Google Scholar]
- 49.Magge DR, Zenati MS, Hamad A, Rieser C, Zureikat AH, Zeh HJ, Hogg ME. Comprehensive comparative analysis of cost-effectiveness and perioperative outcomes between open, laparoscopic, and robotic distal pancreatectomy. HPB. 2018;20:1172–1180. doi: 10.1016/j.hpb.2018.05.014. [DOI] [PubMed] [Google Scholar]
- 50.Marino MV, Mirabella A, Gomez Ruiz M, Komorowski AL. Robotic-assisted versus laparoscopic distal pancreatectomy: the results of a case-matched analysis from a tertiary care center. Dig Surg. 2020;37:229–239. doi: 10.1159/000501428. [DOI] [PubMed] [Google Scholar]
- 51.Najafi N, Mintziras I, Wiese D, Albers MB, Maurer E, Bartsch DK. A retrospective comparison of robotic versus laparoscopic distal resection and enucleation for potentially benign pancreatic neoplasms. Surg Today. 2020;50:872–880. doi: 10.1007/s00595-020-01966-z. [DOI] [PubMed] [Google Scholar]
- 52.Qu L, Zhiming Z, Xianglong T, Yuanxing G, Yong X, Rong L, Yee LW. Short- and mid-term outcomes of robotic versus laparoscopic distal pancreatosplenectomy for pancreatic ductal adenocarcinoma: a retrospective propensity score-matched study. Int J Surg. 2018;55:81–86. doi: 10.1016/j.ijsu.2018.05.024. [DOI] [PubMed] [Google Scholar]
- 53.Raoof M, Nota C, Melstrom LG, Warner SG, Woo Y, Singh G, Fong Y. Oncologic outcomes after robot-assisted versus laparoscopic distal pancreatectomy: analysis of the national cancer database. J Surg Oncol. 2018;118:651–656. doi: 10.1002/jso.25170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rodriguez M, Memeo R, Leon P, Panaro F, Tzedakis S, Perotto O, Varatharajah S, Angelis N, de, Riva P, Mutter D, Navarro F, Marescaux J, Pessaux P. Which method of distal pancreatectomy is cost-effective among open, laparoscopic, or robotic surgery? Hepatobiliary. Surg Nutr. 2018;7:345–352. doi: 10.21037/hbsn.2018.09.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ryan CE, Ross SB, Sukharamwala PB, Sadowitz BD, Wood TW, Rosemurgy AS. Distal pancreatectomy and splenectomy: a robotic or LESS approach. JSLS. 2015;19:e2014–00246. doi: 10.4293/JSLS.2014.00246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Souche R, Herrero A, Bourel G, Chauvat J, Pirlet I, Guillon F, Nocca D, Borie F, Mercier G, Fabre JM. Robotic versus laparoscopic distal pancreatectomy: a French prospective single-center experience and cost-effectiveness analysis. Surg Endosc. 2018;32:3562–3569. doi: 10.1007/s00464-018-6080-9. [DOI] [PubMed] [Google Scholar]
- 57.Vicente E, Nunez-Alfonsel J, Ielpo B, Ferri V, Caruso R, Duran H, Diaz E, Malave L, Fabra I, Pinna E, Isernia R, Hidalgo A, Quijano Y. A cost-effectiveness analysis of robotic versus laparoscopic distal pancreatectomy. Int J Med Robot Computer Assist Surg. 2020;16:e2080. doi: 10.1002/rcs.2080. [DOI] [PubMed] [Google Scholar]
- 58.Waters JA, Canal DF, Wiebke EA, Dumas RP, Beane JD, Aguilar-Saavedra JR, Ball CG, House MG, Zyromski NJ, Nakeeb A, Pitt HA, Lillemoe KD, Schmidt CM. Robotic distal pancreatectomy: cost effective? Surgery. 2010;148:814–823. doi: 10.1016/j.surg.2010.07.027. [DOI] [PubMed] [Google Scholar]
- 59.Xourafas D, Ashley SW, Clancy TE. Comparison of Perioperative Outcomes between Open, Laparoscopic, and Robotic Distal Pancreatectomy: an Analysis of 1815 Patients from the ACS-NSQIP Procedure-Targeted Pancreatectomy Database. J gastrointest surg. 2017;21:1442–1452. doi: 10.1007/s11605-017-3463-5. [DOI] [PubMed] [Google Scholar]
- 60.Yang SJ, Hwang HK, Kang CM, Lee WJ. Revisiting the potential advantage of robotic surgical system in spleen-preserving distal pancreatectomy over conventional laparoscopic approach. Annals Translational Med. 2020;8:188. doi: 10.21037/atm.2020.01.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang J, Jin J, Chen S, Gu J, Zhu Y, Qin K, Zhan Q, Cheng D, Chen H, Deng X, Shen B, Peng C. Minimally invasive distal pancreatectomy for PNETs: laparoscopic or robotic approach? Oncotarget. 2017;8:33872–33883. doi: 10.18632/oncotarget.17513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hu YH, Qin YF, Yu DD, Li X, Zhao YM, Kong DJ, Jin W, Wang H. Meta-analysis of short-term outcomes comparing robot-assisted and laparoscopic distal pancreatectomy. J Comp Eff Res. 2020;9:201–218. doi: 10.2217/cer-2019-0124. [DOI] [PubMed] [Google Scholar]
- 63.Kamarajah SK, Sutandi N, Robinson SR, French JJ, White SA. Robotic versus conventional laparoscopic distal pancreatic resection: a systematic review and meta-analysis. HPB. 2019;21:1107–1118. doi: 10.1016/j.hpb.2019.02.020. [DOI] [PubMed] [Google Scholar]
- 64.Mavrovounis G, Diamantis A, Perivoliotis K, Symeonidis D, Volakakis G, Tepetes K. Laparoscopic versus robotic peripheral pancreatectomy: a systematic review and meta-analysis. J BUON. 2020;25:2456–2475. [PubMed] [Google Scholar]
- 65.Zhou JY, Xin C, Mou YP, Xu XW, Zhang MZ, Zhou YC, Lu C, Chen RG. Robotic versus laparoscopic distal pancreatectomy: a meta-analysis of short-term outcomes. PLoS ONE. 2016;11:e0151189. doi: 10.1371/journal.pone.0151189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gavriilidis P, Lim C, Menahem B, Lahat E, Salloum C, Azoulay D. Robotic versus laparoscopic distal pancreatectomy—The first meta-analysis. HPB. 2016;18:567–574. doi: 10.1016/j.hpb.2016.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Huang B, Feng L, Zhao J. Systematic review and meta-analysis of robotic versus laparoscopic distal pancreatectomy for benign and malignant pancreatic lesions. Surg Endosc. 2016;30:4078–4085. doi: 10.1007/s00464-015-4723-7. [DOI] [PubMed] [Google Scholar]
- 68.Feng Q, Jiang C, Feng X, Du Y, Liao W, Jin H, Liao M, Zeng Y, Huang J. Robotic versus laparoscopic distal pancreatectomy for pancreatic ductal adenocarcinoma: a systematic review and meta-analysis. Front Oncol. 2021;11:752236. doi: 10.3389/fonc.2021.752236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Guerrini GP, Lauretta A, Belluco C, Olivieri M, Forlin M, Basso S, Breda B, Bertola G, Di Benedetto F. Robotic versus laparoscopic distal pancreatectomy: an up-to-date meta-analysis. BMC Surg. 2017;17:105. doi: 10.1186/s12893-017-0301-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rompianesi G, Montalti R, Ambrosio L, Troisi RI. Robotic versus laparoscopic surgery for spleen-preserving distal pancreatectomies: systematic review and meta-analysis. J Pers Med. 2021;11(6):552. doi: 10.3390/jpm11060552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li BQ, Qiao YX, Li J, Yang WQ, Guo JC. Preservation or ligation of splenic vessels during spleen-preserving distal pancreatectomy: a meta-analysis. J Invest Surg. 2019;32:654–669. doi: 10.1080/08941939.2018.1449918. [DOI] [PubMed] [Google Scholar]
- 72.Maggino L, Malleo G, Bassi C, Vollmer C. Splenectomy during distal pancreatectomy: what are we really doing? Gastroenterology. 2018;154:S–1297. doi: 10.1016/S0016-5085(18)34251-3. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.