Abstract
Objective
To study trends in the incidence of reported pulmonary nodules and stage I lung cancer in chest CT.
Methods
We analyzed the trends in the incidence of detected pulmonary nodules and stage I lung cancer in chest CT scans in the period between 2008 and 2019. Imaging metadata and radiology reports from all chest CT studies were collected from two large Dutch hospitals. A natural language processing algorithm was developed to identify studies with any reported pulmonary nodule.
Results
Between 2008 and 2019, a total of 74,803 patients underwent 166,688 chest CT examinations at both hospitals combined. During this period, the annual number of chest CT scans increased from 9955 scans in 6845 patients in 2008 to 20,476 scans in 13,286 patients in 2019. The proportion of patients in whom nodules (old or new) were reported increased from 38% (2595/6845) in 2008 to 50% (6654/13,286) in 2019. The proportion of patients in whom significant new nodules (≥ 5 mm) were reported increased from 9% (608/6954) in 2010 to 17% (1660/9883) in 2017. The number of patients with new nodules and corresponding stage I lung cancer diagnosis tripled and their proportion doubled, from 0.4% (26/6954) in 2010 to 0.8% (78/9883) in 2017.
Conclusion
The identification of incidental pulmonary nodules in chest CT has steadily increased over the past decade and has been accompanied by more stage I lung cancer diagnoses.
Clinical relevance statement
These findings stress the importance of identifying and efficiently managing incidental pulmonary nodules in routine clinical practice.
Key Points
• The number of patients who underwent chest CT examinations substantially increased over the past decade, as did the number of patients in whom pulmonary nodules were identified.
• The increased use of chest CT and more frequently identified pulmonary nodules were associated with more stage I lung cancer diagnoses.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00330-023-09826-3.
Keywords: Multiple pulmonary nodules; Incidence; Tomography, X-ray computed; Natural language processing
Introduction
Randomized controlled trials for lung cancer screening have provided evidence that lung cancer–related mortality can be significantly reduced when the cancer is detected at an early stage and still appears as a nodular lesion [1, 2]. The outcomes of these trials also underline the importance of accurate detection and management of pulmonary nodules outside a screening setting. Hence, the British Thoracic Society (BTS) [3] and the Fleischner Society [4] have established guidelines for the management of incidental nodules to aid radiologists in their decision-making process and to standardize nodule management.
Despite the existence of these guidelines, little data has been published about the incidence of benign and malignant incidental pulmonary nodules in a routine clinical setting, especially in Europe [5]. In the USA, Gould et al [6] performed a large epidemiological study on the trends of reported pulmonary nodules in chest CT from hospitals and medical offices throughout Southern California between 2006 and 2012. They found that the frequency of nodule identification increased from 24 to 31% for all scans performed. However, most remarkably, the incidence of cancerous nodules remained stable regardless.
It is essential to update our knowledge about the incidence of incidental nodules, taking into account the reported increase in nodule identification rate [6], advances in CT scan technology [7], and increased awareness of the risks of nodules due to the outcomes of lung cancer screening trials. Updated statistics are important in clinical decision-making and risk communication between physicians and patients with incidental nodules [8]. In addition, these statistics could aid shaping the research agenda towards finding solutions to cope with the increasing demand for healthcare [9], such as the implementation of artificial intelligence (AI) solutions.
Therefore, the aim of this study was to conduct a large-scale analysis to examine the trends in the incidence of reported pulmonary nodules in chest CT in two large Dutch hospitals in the period of 2008 to 2019. Nodule incidence was correlated with lung cancer diagnosis to assess the clinical relevance of increased nodule detection.
Materials and methods
Study design and data collection
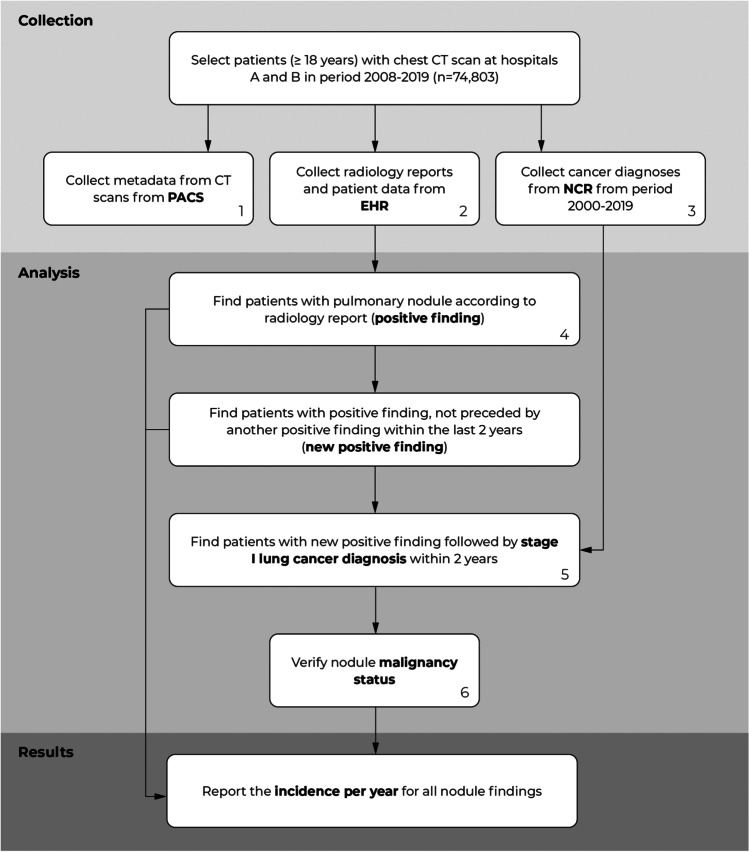
We conducted a retrospective study to identify the incidence of pulmonary nodules in chest CT scans in a university medical center (hospital A) and a large peripheral teaching hospital (hospital B) in the Netherlands. For this purpose, we analyzed the radiology reports from 166,688 chest CT studies and the corresponding lung cancer diagnoses in the years 2008 until 2019 (Table 1). The radiology reports were collected from the Electronic Health Record (EHR) systems. Metadata of the CT scans (i.e., slice thickness) were collected from the Picture Archiving and Communication System (PACS). All cancer diagnoses (pulmonary and extrapulmonary) from the period 2000 to 2019 were obtained from the Netherlands Cancer Registry (NCR), managed by the Netherlands Comprehensive Cancer Organization (IKNL). The NCR comprises all individuals diagnosed with cancer in the Netherlands. At both institutions, the institutional review board waived the need for informed consent because of the retrospective design and the use of anonymized data in this study. A flow diagram of the data collection and analysis procedures is shown in Fig. 1.
Table 1.
Description of the dataset obtained from hospitals A and B, period 2008–2019
| All | Hospital A | Hospital B | ||
|---|---|---|---|---|
| No. of patients | All | 74,803 | 40,440 | 34,363 |
| Men | 40,620 | 22,581 | 18,039 | |
| Women | 34,183 | 17,859 | 16,324 | |
| Patient age1 | All | 60.0 ± 16.0 | 58.1 ± 16.3 | 62.3 ± 15.4 |
| Men | 60.2 ± 16.0 | 58.2 ± 16.4 | 62.7 ± 15.0 | |
| Women | 59.9 ± 16.1 | 58.0 ± 16.1 | 61.9 ± 15.8 | |
| No. of studies | 166,688 | 98,479 | 68,209 | |
| Studies per CT scanner | Siemens Sensation 64 | 48,203 | 13,108 | 35,095 |
| Siemens SOMATOM Definition Flash | 25,695 | 25,695 | ||
| Siemens SOMATOM Definition AS + | 3949 | 3949 | ||
| Siemens SOMATOM Definition Edge | 1987 | 1987 | ||
| Canon Medical Systems Aquilion One | 31,691 | 31,691 | ||
| Siemens Sensation 16 | 28,769 | 28,769 | ||
| Canon Medical Systems Aquilion CXL | 10,278 | 10,278 | ||
| Canon Medical Systems Aquilion Precision | 9286 | 9286 | ||
| Siemens Biograph 40 | 3126 | 3126 | ||
| Other2 | 3704 | 2221 | 1483 |
1Age at the first examination during the investigated period
2CT scanners used in less than 5% of all studies in a single hospital
Fig. 1.
Flow diagram of the data collection and analysis. (1) Netherlands Cancer Registry (NCR). (2) Electronic Health Records (EHR), which stores both radiology reports and patient information (i.e., age, gender). (3) Picture Archiving and Communication System (PACS), which stores all the metadata of the CT scans (i.e., slice thickness, scanner model). (4) Based on a Natural Language Processing (NLP) analysis. (5) Supplementary analysis of other lung cancer stages can be found in Appendix E8 (supplement). (6) The nodule and corresponding lung cancer were manually linked; more details can be found in Appendix E2 (supplement)
Eligibility criteria
All adult patients (≥ 18 years) were included in accordance with the BTS nodule management guideline [3]. We selected patients with at least one CT scan that fully covered the lungs (e.g., CT thorax, CT thorax-abdomen).
Radiology report analysis
NLP algorithm
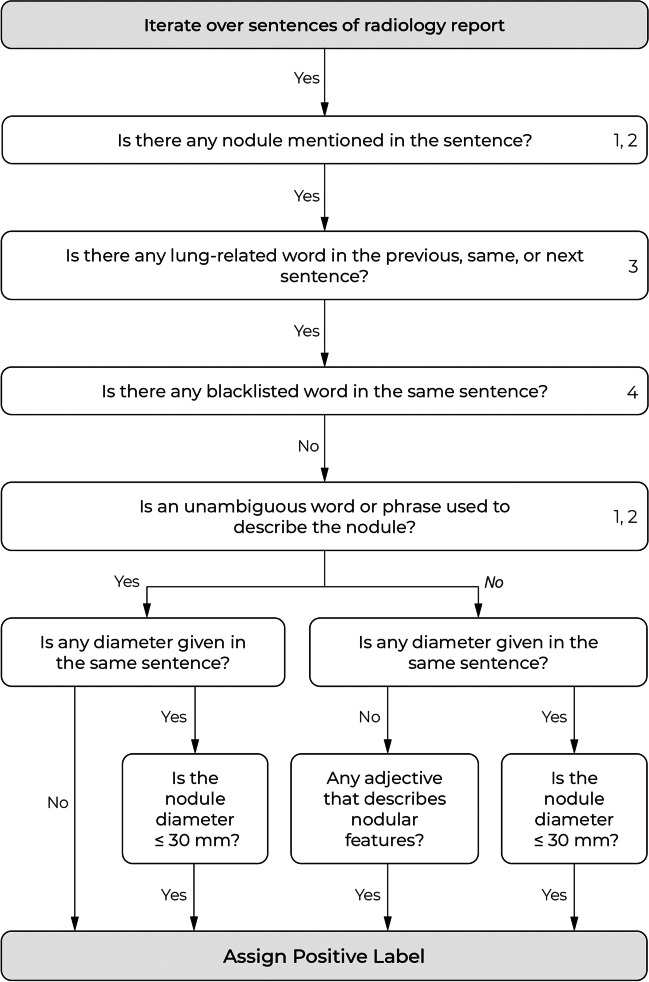
We developed a natural language processing (NLP) algorithm to identify pulmonary nodules described in radiology reports. This algorithm is a rule-based system that uses combinations of keywords (e.g., nodule, lesion) and specific search patterns to find any reported nodule and its diameter. If one or more pulmonary nodules are detected, it returns the largest reported nodule. An overview of the algorithm is shown in Fig. 2. A detailed description of the algorithm and its development is provided in Appendix E1 (supplement).
Fig. 2.
Overview of the natural language processing (NLP) algorithm for the detection of reported pulmonary nodules. The numbers on the right side of a box refer to the lookup tables in Appendix E1 (supplement)
For the development of the NLP algorithm, we created a dataset of 1000 randomly sampled radiology reports from 1000 unique patients from hospital A (n = 500) and hospital B (n = 500). These reports were annotated by W.H. and a medical student under supervision of an experienced radiologist (M.R., 26 years of experience): each report was given a label indicating (1) whether a pulmonary nodule was reported and (2) the diameter of the largest reported nodule if available (otherwise, a missing value was registered). For testing the NLP algorithm, we created an independent dataset of 200 randomly sampled radiology reports from 200 unique patients from hospital A (n = 100) and hospital B (n = 100). There was no overlap between patients from the development and test set. The test set was annotated by an experienced radiologist (E.T.S., 32 years of experience) according to the same annotation procedure as applied for the development set.
We evaluate the NLP algorithm by measuring the sensitivity and specificity for detecting reports with pulmonary nodules on the development and test set. For the subset of true positive detections, we report the sensitivity and specificity for detecting nodule diameter measurements and also report the accuracy for correctly detecting the largest reported nodule diameter.
Analysis and definitions
Per center, we calculated the annual number of chest CTs on study and patient level. A study was marked as “positive” if the report described at least one reported pulmonary nodule with a maximum diameter of 30 mm, regardless of nodule morphology, type (i.e., solid, part-solid, non-solid, calcified, or perifissural), and malignancy status. A study was marked as “new positive” if it was not preceded by another positive study within the last 2 years, which is generally the maximum duration between nodule follow-up examinations [3, 4]. However, this may not fully avoid duplicate counts of rare findings such as persistent subsolid nodules or stable hamartoma [10, 11].
We included the following subanalyses: First, we calculated the number of new nodule findings with a minimum diameter of 5 mm for which follow-up is recommended [3]. These lesions are clinically most relevant and should be measured and reported consistently at hospitals A and B. Second, we calculated the number of new nodule findings per CT protocol (i.e., scanner model, slice thickness). Third, the total number of patients with a new positive finding was calculated for subgroups which were stratified by age and sex. Finally, for each year, a subanalysis was conducted for patients with chest CT scans without a history of malignancy (both extrapulmonary and pulmonary) within the last 10 years. Patients with (prior) cancer are more likely to develop pulmonary nodules (i.e., metastases). Moreover, the yearly influx of non-cancer patients should be tracked in order to properly interpret the results of the lung cancer analysis (see the next section).
Lung cancer analysis
To investigate the relationship between nodule detection and early lung cancer detection, we calculated the incidence of lung cancer diagnoses within 2 years after a new positive chest CT scan. By only taking new positive chest CT scans, duplicate nodule counts were avoided for patients with multiple follow-up examinations over the years. We focused on the trend analysis of stage I cancers, as they include detections of nodules instead of masses (> 30 mm) [12]. For this analysis, newly reported nodules were manually linked to the corresponding stage I cancer diagnoses by an experienced radiologist (E.T.S.). The details of this procedure are provided in Appendix E2 (supplement).
The total number of yearly new positive CT studies with consequent lung cancer diagnosis was calculated and then stratified by cancer stage according to the respective TNM classification at the time of diagnosis (editions 5–8) [12]. Carcinoma in situ or cancers with missing TNM staging were excluded. According to the NCR, lung cancer diagnoses were based on histological examination, cytology testing, or clinical diagnostic testing (e.g., medical imaging, exploratory surgery). An overview of the number of diagnoses per basis is provided in Appendix E3 (supplement).
Results
Evaluation of the NLP algorithm
The algorithm had a sensitivity of 94% (62/66) and specificity of 96% (128/134) for identifying radiology reports with pulmonary nodules in the test set. For the subset of true positive detections, it had a sensitivity of 90% (27/30) and specificity of 97% (31/32) for identifying those with nodule diameter measurements. The algorithm had an accuracy of 93% (25/27) for correctly detecting the largest reported nodule diameter. An overview of the evaluation metrics on the development and test set is provided in Table 2.
Table 2.
Evaluation scores of the NLP algorithm on the development and test dataset
| Development dataset | Test dataset | ||
|---|---|---|---|
| Number of reports | All | 1000 | 200 |
| Hospital A | 500 | 100 | |
| Hospital B | 500 | 100 | |
| Positive labels (n, % of all positive labels) | All | 348 | 66 |
| With nodule diameter | 186 (53%) | 32 (48%) | |
| Without nodule diameter | 162 (47%) | 34 (52%) | |
| Negative labels | 652 | 134 | |
| Detecting nodules (%, proportion) | Sensitivity | 94.0 (327/348) | 93.9 (62/66) |
| Specificity | 96.3 (628/652) | 95.5 (128/134) | |
| Detecting nodule diameter1 (%, proportion) | Sensitivity | 92.2 (165/179) | 90.0 (27/30) |
| Specificity | 98.0 (144/148) | 96.9 (31/32) | |
| Detecting largest nodule diameter2 (%, proportion) | Accuracy | 97.6 (161/165) | 92.6 (25/27) |
1Only applicable to the subset of true positive reports
2Only applicable to the subset of true positive reports with nodule diameter measurements
Radiology report analysis
Results on scan level
Between 2008 and 2019, 166,688 chest CT studies were performed in hospitals A and B (Table 1). From these studies, 98,479 chest CT studies were conducted in hospital A and 68,209 in hospital B (Table 1). The total annual number of chest CT studies more than doubled from 9955 in 2008 to 20,476 in 2019 (Table 3). The average number of chest CT scans per patient only slightly increased from 1.45 in 2008 to 1.54 in 2019.
Table 3.
Annual number of positive chest CT scans in hospitals A and B, patient and scan-level data (2008–2019)
| Patients with positive finding (n (%) of total patients) | Positive studies (n (%) of total studies) | |||||
|---|---|---|---|---|---|---|
| Year | Total patients | Any nodule1 | Nodule with diameter ≥ 5 mm | Total studies | Any nodule1 | Nodule with diameter ≥ 5 mm |
| 2008 | 6845 | 2595 (37.9) | 994 (14.5) | 9955 | 3806 (38.2) | 1362 (13.7) |
| 2009 | 6986 | 2494 (35.7) | 925 (13.2) | 10,019 | 3611 (36.0) | 1294 (12.9) |
| 2010 | 7477 | 2738 (36.6) | 1017 (13.6) | 10,426 | 3901 (37.4) | 1339 (12.8) |
| 2011 | 7574 | 2889 (38.1) | 1173 (15.5) | 10,613 | 4090 (38.5) | 1552 (14.6) |
| 2012 | 8117 | 3262 (40.2) | 1307 (16.1) | 11,328 | 4538 (40.1) | 1685 (14.9) |
| 2013 | 8910 | 3748 (42.1) | 1694 (19.0) | 12,567 | 5420 (43.1) | 2311 (18.4) |
| 2014 | 9711 | 4234 (43.6) | 1969 (20.3) | 13,943 | 6160 (44.2) | 2696 (19.3) |
| 2015 | 10,659 | 4895 (45.9) | 2283 (21.4) | 15,516 | 7218 (46.5) | 3234 (20.8) |
| 2016 | 10,964 | 5281 (48.2) | 2616 (23.9) | 15,823 | 7725 (48.8) | 3667 (23.2) |
| 2017 | 11,536 | 5849 (50.7) | 3004 (26.0) | 17,007 | 8828 (51.9) | 4224 (24.8) |
| 2018 | 12,689 | 6268 (49.4) | 3032 (23.9) | 19,015 | 9562 (50.3) | 4211 (22.1) |
| 2019 | 13,286 | 6654 (50.1) | 3108 (23.4) | 20,476 | 10,523 (51.4) | 4407 (21.5) |
1Also includes pulmonary nodules without reported diameter
During the same period, the total number of positive chest CT studies increased from 3806 (38%) in 2008 to 10,523 (51%) in 2019 (Table 3). For positive findings with a minimum nodule diameter of 5 mm, the total number of positive chest CT studies tripled from 1362 (14%) in 2008 to 4407 (22%) in 2019. The percentage of positive chest CT studies reached a plateau in 2017 and then remained constant in both hospitals. The trend analyses per hospital are included in Appendix E4 (supplement). The incidence of new positive chest CT studies per CT protocol is provided in Appendix E5 (supplement).
Results on patient level
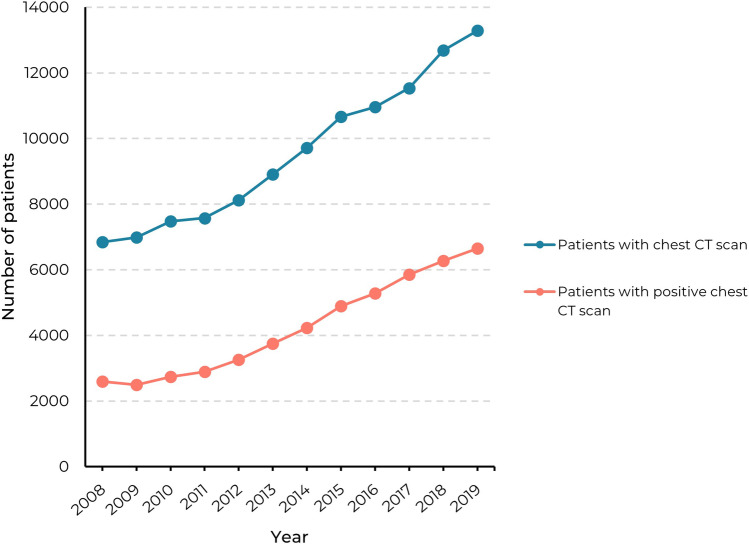
In hospitals A and B, 74,803 unique patients underwent a chest CT scan between 2008 and 2019 (Table 1). From these patients, 40,440 patients were examined in hospital A and 34,363 patients in hospital B (Table 1). The total number of patients who underwent a chest CT scan doubled from 6845 in 2008 to 13,286 in 2019 (Table 3). The total number of patients with a positive finding increased from 2595 (38%) in 2008 to 6654 (50%) in 2019. In Fig. 3, this trend is visualized and compared with the total annual number of patients who underwent a chest CT scan. For positive findings with a minimum nodule diameter of 5 mm, the total number of patients with a positive finding tripled from 994 (15%) in 2008 to 3108 (23%) in 2019. The mean age of patients who underwent a chest CT scan linearly increased from 58.1 ± 15.2 years in 2008 to 62.6 ± 14.3 years in 2019.
Fig. 3.
Annual number of unique patients with a (positive) chest CT scan in hospitals A and B in the period 2008–2019
The total annual number of patients with a new positive finding more than doubled from 2006 (out of 6954, 29%) in 2010 to 4107 (out of 11,258, 37%) in 2019 (Table 4). For new positive findings with a minimum nodule diameter of 5 mm, the total number of patients increased from 608 (9%) in 2010 to 1611 (14%) in 2019. A subanalysis of the patients without any prior cancer diagnosis can be found in Appendix E6 (supplement). The yearly percentage of this group remained constant during the investigated period (56.0 ± 1.5%). An overview of the nodule size distribution per year is included in Appendix E7 (supplement).
Table 4.
Annual number of patients with new positive chest CT scans and those followed by lung cancer diagnosis within 2 years in hospitals A and B (2010–2019)
| Patients with new positive chest CT scan (n, % of total patients)1 | Patients with new positive chest CT scan and subsequent stage I lung cancer diagnosis within 2 years (n, % of total patients)1 | |||
|---|---|---|---|---|
| Year | Total patients1 | Any nodule2 | Nodule with diameter ≥ 5 mm | Any nodule3 |
| 2010 | 6954 | 2006 (28.8) | 608 (8.7) | 26 (0.4) |
| 2011 | 7021 | 2100 (29.9) | 743 (10.6) | 30 (0.4) |
| 2012 | 7570 | 2451 (32.4) | 837 (11.1) | 33 (0.4) |
| 2013 | 8147 | 2736 (33.6) | 1056 (13.0) | 49 (0.6) |
| 2014 | 8848 | 3031 (34.3) | 1186 (13.4) | 63 (0.7) |
| 2015 | 9467 | 3382 (35.7) | 1325 (14.0) | 65 (0.7) |
| 2016 | 9564 | 3518 (36.8) | 1462 (15.3) | 73 (0.8) |
| 2017 | 9883 | 3812 (38.6) | 1660 (16.8) | 78 (0.8) |
| 2018 | 10,890 | 4012 (36.8) | 1613 (14.8) | NA |
| 2019 | 11,258 | 4107 (36.5) | 1611 (14.3) | NA |
1Excluded all patients with a positive chest CT scan within the previous 2 years
2Also includes pulmonary nodules without reported diameter
3Nodule and corresponding lung cancer location were manually verified (see Appendix E2)
Analysis of subgroups with respect to age
The incidence of new nodule findings increased with age until the age of 70 years (Fig. 4): from 486 (23%) in the age group of 18 to 24 years (n = 2120) to 5014 (47%) in the age group of 65 to 69 years (n = 10,764). After the age of 70 years, the incidence decreased: from 4514 (46%) in the age group of 70 to 74 years (n = 9819) to 121 (25%) in the age group of 90 years or older (n = 476). New nodules were more frequently reported in men in comparison to women in all age groups (16,383 men versus 12,975 women), except in the age group of 90 years or older.
Fig. 4.
The proportion of patients with a new positive chest CT scan in hospitals A and B in the period 2010–2019, grouped by age and sex. The denominator is the number of patients with a chest CT per age group. Only the first new positive finding of a patient is counted
Lung cancer analysis
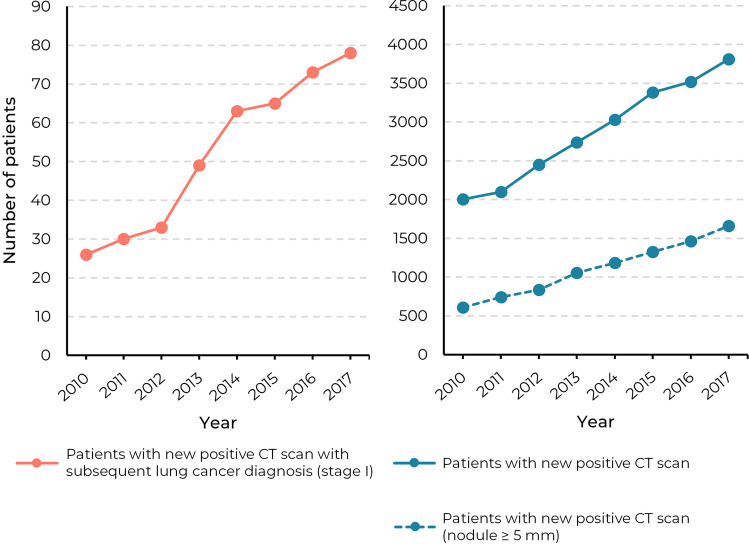
The total annual number of patients with a new positive finding with a subsequent stage I lung cancer diagnosis increased from 26 (out of 6954, 0.4%) in 2010 to 78 (out of 9883, 0.8%) in 2017 (Table 4). In Fig. 5, this trend is visualized and compared with the total annual number of patients with a new positive finding. A subanalysis of the patients without a prior cancer diagnosis can be found in Appendix E6 (supplement). The analyses for lung cancer stages II, III, and IV are included in Appendix E8 (supplement).
Fig. 5.
The number of patients with a new positive CT scan (all nodules and nodules ≥ 5 mm) compared with those with a subsequent stage I lung cancer diagnosis within 2 years in hospitals A and B in the period 2010–2017
Discussion
In this retrospective study, we analyzed an extensive dataset of radiology reports and lung cancer diagnoses records from two Dutch hospitals, one academic and one teaching hospital, over a period of 12 years. Radiology reports were analyzed using a validated NLP algorithm. In the period between 2008 and 2019, we found that the annual number of chest CT studies doubled and the number of positive studies almost tripled. The nodule incidence increased from 38 to 51% in all chest CT studies. The number of patients in whom new pulmonary nodules were reported also doubled in the period between 2010 and 2019. By linking our results to the Netherlands Cancer Registry, we also found that the more frequent identification of pulmonary nodules was accompanied by additional cases of stage I lung cancer diagnoses.
There are various potential causes that may explain the trends that we found. The positive results from lung cancer screening trials and the aging patient population have probably contributed to the more frequent identification of pulmonary nodules. Results from large lung cancer screening trials such as NLST [2] and NELSON [1] were published during the investigated period and likely raised awareness among radiologists for the potential risks of pulmonary nodules and the need of monitoring for early lung cancer detection. Another explanation could be the aging patient population, considering that the nodule incidence increases with age (until the age of 70 years), as supported by our analysis.
It is uncertain whether improvements in the quality of the performed chest CT scans contributed to the increased nodule incidence: the 16- and 64-slice CT scanners were gradually phased out and replaced by newer 128-slice, 160-slice, and 320-slice CT scanners at both hospitals between 2011 and 2017. At hospital A, we found a higher nodule incidence for CT scans with low slice thickness (< 1 mm) from the newer scanners compared to scans with a higher slice thickness (≥ 3 mm) from the older scanners (Appendix E5, supplement). At hospital B, we conversely found a higher nodule incidence in scans with a high slice thickness (≥ 3 mm) from all scanners. Therefore, advances in CT technology may have contributed in hospital A, but probably not in hospital B.
We found that the trends in the annual number of CT studies and (new) positive CT studies were largely similar between hospitals A and B. It is noticeable that the percentage of positive studies reached a plateau in 2017 in both hospitals. A possible explanation is the publication of the BTS guidelines in 2015 [3], which could have resulted in fewer reported small (≤ 5 mm) or clearly benign nodules. Furthermore, the updated Fleischner nodule management guidelines were later published in 2017, in which the minimal threshold size for follow-up of solitary solid nodules was increased from 4 to 6 mm [4]. When comparing the overall nodule incidence between the hospitals, the nodule incidence was substantially higher in hospital A as compared to that in hospital B (44% vs. 29% in 2008, 59% vs. 41% in 2019). This may be explained by the fact that hospital A is an academic hospital, where pulmonary nodules are more researched. Furthermore, the impact of institution (academic versus non-academic) as well as CT technology is most likely influenced by the individual readers’ performance [13].
Our findings correspond to those from Gould et al [6] who reported increases in the identification of pulmonary nodules in chest CT more than a decade ago. They found that pulmonary nodules with a minimum diameter of 4 mm were identified in 29% of all scans performed between 2006 and 2012. For any reported nodule, our estimate is 45% for all scans performed between 2008 and 2019 and 19% when using a nodule diameter threshold of 5 mm. A key difference in our findings is that Gould et al found that the increases in nodule detection did not identify additional cases of lung cancer, whereas we found that increased nodule identifications were associated with more stage I lung cancer diagnoses within 2 years. The latter might be among other factors attributable to the results of the NLST trial in 2011 [2] that has led to increased knowledge but also higher awareness of the importance of small nodules for the detection of stage I cancers.
This study has several limitations. First, our NLP algorithm takes all pulmonary nodules into consideration and cannot rule out nodules that were described with typical benign features (e.g., calcified or perifissural nodules or micronodules). An increase of these nodules would not lead to additional CT follow-up [3, 4]. However, the diameter of these low-risk nodules is typically not measured and therefore, these lesions were largely ignored in our subanalyses where a diameter threshold of 5 mm was applied. Second, an automated analysis of free-text radiology reports may not give the most accurate estimation of the incidence of pulmonary nodules due to inter- and intra-observer variability among reading radiologists. A more accurate method could be an automated analysis of the CT scans with AI systems, although this would require a substantial CT database, a clinically validated AI system, and a significant amount of computing resources. Finally, CT scans that only contain portions of the lungs (e.g., CT scan of the abdomen, neck, or heart) were not included in the analysis. However, the analysis of chest CT scans should already include the vast majority of the reported incidental pulmonary nodules in clinical practice.
In conclusion, we observed that the number of patients who underwent chest CT examinations substantially increased over the past decade, as did the number of patients in whom a pulmonary nodule was identified. The more frequent pulmonary nodule identifications were associated with more stage I lung cancer diagnoses. The results need to be validated in a larger, geographically diverse cohort. However, these preliminary findings suggest that the more frequent chest CT scans and incidental nodule identifications lead to an increased detection of early-stage lung cancer, and stress the importance of efficient nodule management.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank the registration team of the Netherlands Comprehensive Cancer Organization (IKNL) for the collection of data for the Netherlands Cancer Registry as well as IKNL staff for scientific advice. We thank Karlijn Rutten for her help in the annotation of radiology reports. We thank Tijs Samson for his support in the data acquisition from the hospital information systems.
Abbreviations
- AI
Artificial intelligence
- BTS
British Thoracic Society
- EHR
Electronic Health Record
- IKNL
Netherlands Comprehensive Cancer Organization
- NCR
Netherlands Cancer Registry
- NLP
Natural language processing
- PACS
Picture Archiving and Communication System
- TNM
TNM Classification of Malignant Tumours
Funding
This work was supported by the Junior Researcher grant from the Radboud Institute for Health Sciences, Radboudumc, Nijmegen, the Netherlands, and the Jeroen Bosch Hospital, Den Bosch, the Netherlands.
Declarations
Guarantor
The scientific guarantor of this publication is Colin Jacobs.
Conflict of interest
C. Jacobs is a member of the European Radiology Scientific Editorial Board. He has not taken part in the review or selection process of this article.
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Institutional Review Board approval was obtained.
Study subjects or cohorts overlap
Not applicable.
Methodology
• Retrospective
• Observational
• Multicenter study
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.de Koning HJ, van der Aalst CM, de Jong PA, et al. Reduced lung-cancer mortality with volume CT screening in a randomized trial. N Engl J Med. 2020 doi: 10.1056/NEJMoa1911793. [DOI] [PubMed] [Google Scholar]
- 2.The National Lung Screening Trial Research Team Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011 doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Callister MEJ, Baldwin DR, Akram AR, et al. British Thoracic Society guidelines for the investigation and management of pulmonary nodules. Thorax. 2015 doi: 10.1136/thoraxjnl-2015-207168. [DOI] [PubMed] [Google Scholar]
- 4.MacMahon H, Naidich DP, Goo JM, et al. Guidelines for management of incidental pulmonary nodules detected on CT images: from the Fleischner Society 2017. Radiology. 2017 doi: 10.1148/radiol.2017161659. [DOI] [PubMed] [Google Scholar]
- 5.Schmid-Bindert G, Vogel-Claussen J, Gütz S, et al. Incidental pulmonary nodules – what do we know in 2022. Respiration. 2022 doi: 10.1159/000526818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gould MK, Tang T, Liu I-LA, et al. Recent trends in the identification of incidental pulmonary nodules. Am J Respir Crit Care Med. 2015 doi: 10.1164/rccm.201505-0990OC. [DOI] [PubMed] [Google Scholar]
- 7.Agostini A, Borgheresi A, Granata V, et al. Technological advances in body CT: a primer for beginners. Eur Rev Med Pharmacol Sci. 2022 doi: 10.26355/eurrev_202211_30144. [DOI] [PubMed] [Google Scholar]
- 8.Wiener RS, Gould MK, Woloshin S, Schwartz LM, Clark JA (2013) What do you mean, a spot? A qualitative analysis of patients’ reactions to discussions with their physicians about pulmonary nodules. Chest. 10.1378/chest.12-1095 [DOI] [PMC free article] [PubMed]
- 9.Bruls RJM, Kwee RM. Workload for radiologists during on-call hours: dramatic increase in the past 15 years. Insights Imaging. 2020 doi: 10.1186/s13244-020-00925-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mets OM, de Jong PA, Scholten ET, Chung K, van Ginneken B, Schaefer-Prokop CM. Subsolid pulmonary nodule morphology and associated patient characteristics in a routine clinical population. Eur Radiol. 2017 doi: 10.1007/s00330-016-4429-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Siegelman SS, Khouri NF, Scott W, et al. Pulmonary hamartoma: CT findings. Radiology. 1986 doi: 10.1148/radiology.160.2.3726106. [DOI] [PubMed] [Google Scholar]
- 12.Brierley JD, Gospodarowicz MK, Wittekind C. TNM classification of malignant tumours. 8. Hoboken, USA: John Wiley & Sons Inc; 2016. [Google Scholar]
- 13.Pinsky PF, Gierada SD, Hrudaya NP, Kazerooni E, Amorosa J. National lung screening trial: variability in nodule detection rates in chest CT studies. Radiology. 2013 doi: 10.1148/radiol.13121530. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.