Abstract
Background
Venous thrombosis is a common complication after hip arthroplasty.
Methods
PubMed, Embase, the Cochrane Library, and Web of Science were searched for case-control studies on risk factors for venous thrombosis after hip arthroplasty up to September 14, 2023. Data analysis was performed using Stata15.0.
Results
This meta-analysis included 15 case-control studies, comprising 17,909 participants, including 1149 patients with venous thrombosis and 16,760 patients without venous thrombosis. The univariate meta-analysis results revealed that age over 70 years old, hypertension, BMI ≥ 25, operation time, stroke, and use of mechanical prophylaxis alone were risk factors for venous thrombosis after hip arthroplasty. Multivariate analysis showed that female, age > 70 years, BMI ≥ 25, cemented prosthesis, and a history of venous thrombosis were the risk factors for venous thrombosis after hip arthroplasty.
Conclusions
This study suggests that female sex, age over 70 years, hypertension, a BMI of 25 or higher, duration of operation, stroke, mechanical prevention only, use of a cemented prosthesis, and a history of venous thrombosis are risk factors for venous thrombosis following hip arthroplasty.
Clinical trial number
not applicable.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12891-025-08764-z.
Keywords: Hip arthroplasty, Risk factors, Meta-Analysis, Systematic review, Venous thrombosis
Background
As the population ages, the incidence of hip fractures, osteonecrosis of the femoral head, and osteoarthritis in older people is progressively increasing. Conservative treatments have proven ineffective in relieving pain in this population. Hip arthroplasty (HA) has emerged as a viable surgical solution for treating end-stage hip diseases in older patients. It can alleviate pain, enhance joint function, and rectify deformities. Currently, it is considered one of the primary surgical methods to treat these conditions, enhancing hip function and improving quality of life [1, 2]. It is projected that the demand for HA will nearly triple by 2030 [3]. Venous thromboembolism (VTE) is a common complication of HA, and there are multiple risk factors for VTE [4]. VTE is a severe stress response with a high incidence [5, 6]. This condition clinically manifests as sudden limb swelling, venous thrombosis of the lower extremity, and localized pain, and it may become more severe when patients walk. Venous thrombosis refers to abnormal coagulation of blood in veins, leading to venous lumen obstruction and thrombus formation [7]. Furthermore, most thrombi will shed and spread with blood flow to the main deep vein, forming deep vein thrombosis. If not promptly treated, it would induce pulmonary embolism in a majority of patients, and approximately 10% of them will die within an hour [8]. This disease poses significant physical harm and threatens the life of the affected patients [9].
The specific pathogenesis of venous thrombosis following HA is complex and remains unclear [10, 11]. Although HA can improve the quality of life for older patients with end-stage hip diseases, venous thrombosis after surgery significantly hampers their quality of life [12]. Currently, our understanding of the pathophysiological, biochemical, molecular, and pharmacological mechanisms underlying postoperative venous thrombosis is incomplete. Patients with venous thrombosis often experience slow recovery, increased treatment costs, poor clinical efficacy, and even life-threatening complications, necessitating careful attention [13]. Therefore, identifying the influencing factors of venous thrombosis after HA is imperative. However, the risk factors for venous thrombosis in patients undergoing HA remain debatable [14]. Hence, this study aimed to explore the risk factors for venous thrombosis after HA. The ultimate goal is to promote the prevention and treatment of postoperative thrombosis, and enhance the quality of life and prognosis of patients after HA.
Materials and methods
The study was reported according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines [15]. The study protocol was registered under No. CRD42023476372. This study did not involve humans, and thus IRB approval was not required.
Literature search
Case-control studies examining risk factors for venous thrombosis following HA were systematically searched in databases including PubMed, Embase, the Cochrane Library, and Web of Science up to September 14, 2023. The following subject and free words were employed in the search process: “arthroplasty”, “replacement”, “hip”, “venous thrombosis”, and “risk factors”. Detailed search strategies are provided in Supplementary material 1.
Inclusion and exclusion criteria
Inclusion criteria were as follows: Patients who underwent HA [16], and the exposure was venous thrombosis. The diagnostic criteria for venous thrombosis here were deep vein thrombosis (proximal, distal) and pulmonary embolism detected by color Doppler ultrasound, spiral computed tomography pulmonary angiography (PE), and perfusion/ventilation scintigraphy. The study design must be a retrospective cohort study. The primary outcome measures were single- and multivariate analyses of risk factors, regardless of univariate or multivariate analysis. However, when both univariate and multivariable analysis results were reported in a study, the multivariate results were given priority, and the multivariate-adjusted risk value was extracted. If only univariate analysis was performed, the univariate analysis results were extracted.
The following studies were excluded: conference abstracts, meta-analyses, letters, duplicate published articles, systematic reviews, studies with unavailable full texts and unextractable data, and animal experiments.
Data extraction
Two independent reviewers independently screened the searched studies. They read titles and abstracts to directly select potentially eligible studies. Then the full texts were downloaded and read to select eligible studies. In the screening process, the inclusion and exclusion criteria were strictly followed. Relevant data were extracted, and the extracted data were cross-checked to ensure consistency. The main extracted data included the first author’s name, publication year, country, study design, sample size, sex, and age.
Evaluation of quality
The Newcastle-Ottawa Scale (NOS) [17] was used to evaluate the case-control studies, including three aspects: selection of study population (4 points), comparability between groups (2 points), and exposure factors or outcome measures (3 points). The scale has a total score of 9 points, with a score of ≤ 4 considered as low quality, 5–6 as medium quality, and ≥ 7 as high quality. If there were dissents between the two researchers during the evaluation process, they would discuss to reach a consensus, or a third party would be consulted.
Statistical analysis
Stata15.0 was used to analyze the data. Since all the retrospective cohort studies included in this analysis provided odds ratio (OR) values, we uniformly used OR as effect size. The pooled OR value and 95%CI were calculated. Continuous variables were expressed by weighted mean difference (WMD) with 95% confidence interval (CI, range of values for the estimated parameter). It was used in meta-analysis where all included studies meet two conditions: the same continuous outcome variable and unit of measurement. Dichotomous variables were expressed by OR with 95% CI (mainly used in retrospective case-control studies to establish the association between an event outcome and a factor by obtaining data from both the case and control groups to study the correlation between the two). According to the results of the heterogeneity test (Q-test, mainly used to make trade-off judgments on suspicious values.) and I2 statistic, a corresponding model was selected to calculate the pooled OR value. If I2 > 50%, the random-effects model was used, and if I2 ≤ 50%, the fixed-effects model was used. When I2 > 50%, the sensitivity analysis was performed by excluding the studies one by one. Egger’s test was used to analyze the publication bias, with the test level α = 0.05. P < 0.05 was considered statistically significant.
Result
Literature screening process and results
By searching PubMed, Embase, the Cochrane Library and Web of Science databases, a total of 8,099 articles were initially identified. Following the removal of duplicate articles, 965 studies remained. After reading the titles and abstracts, 72 articles were left. After a full-text review, 15 studies were ultimately included. The detailed literature screening process is illustrated in Fig. 1.
Fig. 1.
Literature search flow chart
Since the sample size from the study by Keller et al. [18] accounted for a very high proportion of the combined sample size (about 99%), in order to ensure the robustness of the study results and balance the weights between different studies, thereby improving the generalizability of the results, it was necessary to reduce the excessive impact of a single study on the overall results. Hence, we excluded their study. After excluding their study, in the multivariate meta-analysis, the P value for female changed from 0.0001 to 0.001, while the P value for age > 70 remained unchanged (P = 0.0001). In the univariate meta-analysis, the P value for hypertension did not change (P = 0.001), while the P value for stroke changed from 0.015 to 0.006. The results of these factors were not affected by the exclusion of the study by Keller et al. Nonetheless, in the univariate meta-analysis results, the P value for major bleeding or transfusion of blood changed from 0.041 to 0.162, while the P value for coronary artery disease changed from 0.0001 to 0.122. Consistent evidence for these factors in smaller, more homogeneous cohorts was lacking, and hence, these results should be interpreted with caution. We ultimately decided to exclude Keller’s study in order to present globally generalizable risk factors rather than results driven by a single dataset (Supplementary files 1–2).
Basic characteristics of the included literature
All the 15 included studies were case-control studies [19–33], and nine of them were from China [33]. There were 1149 HA patients with venous thrombosis and 16,760 HA patients without venous thrombosis. The mean age of the patients ranged from 62.5 to 69 years old. The specific characteristics of the included studies are shown in Table 1. The quality of the 15 articles was assessed using the NOS. All studies had an NOS score of 8–9, and the overall quality of the included studies was high. The specific quality assessment is shown in Table 2.
Table 1.
Table of literature characteristics
| Study | Year | Country | Sample size | VT sample size | Mean age | Gender(M/F) | Risk factors |
|---|---|---|---|---|---|---|---|
| Deng W | 2021 | China | 400 | 187 | 64.9 | 97/303 | logistic |
| Fujita Y | 2015 | Japan | 1163 | 51 | 69 | 168/995 | Logistic |
| Howard T A | 2022 | UK | 2740 | 34 | 1089/1651 | logistic | |
| Jiang T | 2019 | China | 715 | 57 | 65.1 | 208/507 | logistic |
| Migita K | 2014 | Japan | 868 | 109 | 128/740 | logistic | |
| Shimoyama Y | 2012 | Japan | 144 | 61 | 18/126 | logistic | |
| Tang J | 2018 | China | 214 | 23 | 78/136 | logistic | |
| White R H | 2000 | USA | 889 | 297 | 325/564 | bivariate analysis | |
| Xu H | 2019 | China | 365 | 73 | 16/57 | logistic | |
| Xu Z | 2016 | China | 224 | 30 | 96/128 | logistic | |
| Yao Y | 2018 | China | 402 | 78 | 63.55 | 78/324 | Logistic |
| Yu X | 2021 | China | 182 | 36 | 112/70 | logistic | |
| Yukizawa Y | 2019 | Japan | 419 | 44 | 62.5 | 102/317 | logistic |
| Zeng Y | 2019 | China | 9022 | 19 | 63.6 | 4040/4982 | Logistic |
| Zhang S | 2015 | China | 162 | 50 | 71/91 | Logistic |
Table 2.
NOS scores
| Study | Is the case definition adequate? | Representativeness of the cases | Determination of control group | Definition of Controls | Comparability of cases and controls on the basis of the design or analysis | Ascertainment of exposure | Same method of ascertainment for cases and controls | Non response | Total scores |
|---|---|---|---|---|---|---|---|---|---|
| Deng W 2021 | * | * | * | * | ** | * | * | * | 9 |
| Fujita Y 2015 | * | * | * | * | * | * | * | * | 8 |
| Howard T A 2022 | * | * | * | * | ** | * | * | * | 9 |
| Jiang T 2019 | * | * | * | * | * | * | * | * | 8 |
| Migita K 2014 | * | * | * | * | * | * | * | * | 8 |
| Shimoyama Y 2012 | * | * | * | * | * | * | * | * | 8 |
| Tang J 2018 | * | * | * | * | ** | * | * | * | 9 |
| White R H 2000 | * | * | * | * | ** | * | * | * | 9 |
| Xu H 2019 | * | * | * | * | * | * | * | * | 8 |
| Xu Z 2016 | * | * | * | * | ** | * | * | * | 9 |
| Yao Y 2018 | * | * | * | * | * | * | * | * | 8 |
| Yu X 2021 | * | * | * | * | * | * | * | * | 8 |
| Yukizawa Y 2019 | * | * | * | * | ** | * | * | * | 9 |
| Zeng Y 2019 | * | * | * | * | * | * | * | * | 8 |
| Zhang S 2015 | * | * | * | * | * | * | * | * | 8 |
Univariate meta-analysis results
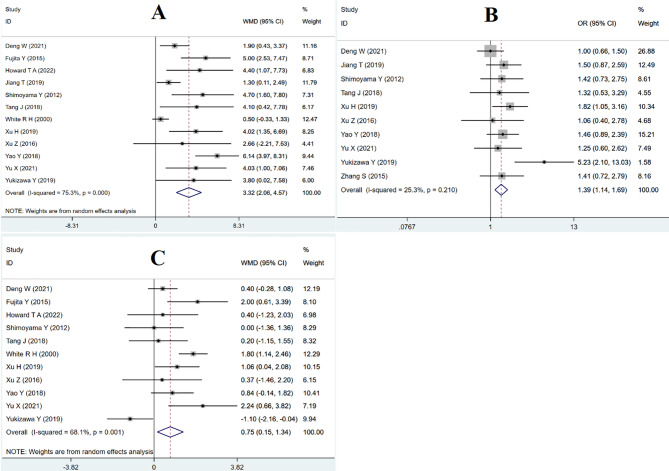
The univariate analysis results were subsequently analyzed and pooled. The findings indicated that male sex was a protective factor against venous thrombosis after HA. However, factors such as female sex, smoke, hyperlipidemia, preoperative D-dimer > 0.55, varicose veins, hemoglobin, types of anesthesia, cancer, coronary artery disease, diabetes, history of cerebrovascular disease, major bleeding or transfusion, and intraoperative blood loss were not found to be significantly associated with the risk of venous thrombosis following HA (Table 3). Factors contributing to the risk of venous thrombosis post-HA included age > 70 years [WMD = 3.32, 95% CI (2.06, 4.57), P = 0.0001] (Fig. 2A; Table 3), hypertension [OR = 1.39, 95% CI (1.14, 1.69), P = 0.001] (Fig. 2B; Table 3), BMI ≥ 25 [WMD = 0.75, 95% CI (0.15, 1.34), P = 0.014] (Fig. 2C; Table 3), the duration of surgery [WMD=-18.35, 95% CI (-35.34, -1.35), P = 0.034] (Fig. 3A; Table 3), stroke [OR = 1.95, 95% CI (1.21, 3.13), P = 0.006] (Fig. 3B; Table 3), and exclusive use of mechanical prophylaxis such as compression stockings or a foot pump [OR = 0.65, 95% CI(0.51, 0.82), P = 0.0001] (Fig. 3C; Table 3). The sensitivity analysis results are shown in Supplementary Figs. 1–3.
Table 3.
Univariate meta-analysis
| Risk factors | No of study | Heterogeneity | OR/WMD (95%CI) | P | Egger | Grade rating | |
|---|---|---|---|---|---|---|---|
| I2 (%) | P | ||||||
| Male | 15 | 70.4 | 0.000 | 0.74 (0.54, 1.01) | 0.062 | 0.342 | Moderate |
| Female | 15 | 70.4 | 0.000 | 1.35(0.99, 1.84) | 0.062 | 0.342 | Moderate |
| Age>70(continuous) | 12 | 75.3 | 0.000 | 3.32(2.06, 4.57) | 0.0001 | 0.001 | Moderate |
| smoke | 5 | 0 | 0.590 | 0.86 (0.61, 1.20) | 0.372 | 0.738 | low |
| Hypertension | 10 | 25.3 | 0.210 | 1.39 (1.14, 1.69) | 0.001 | 0.208 | Moderate |
| Cerebrovascular disease | 3 | 82.3 | 0.003 | 2.46(0.49, 12.42) | 0.276 | 0.589 | low |
| BMI ≥ 25 (continuous) | 11 | 68.1 | 0.001 | 0.75 (0.15, 1.34) | 0.014 | 0.645 | Moderate |
| Hyperlipidemia | 3 | 52.7 | 0.121 | 1.65 (0.60, 4.51) | 0.331 | 0.333 | low |
| Preoperative D-dimer > 0.55 | 5 | 62.2 | 0.032 | 0.30(-0.01,0.61) | 0.055 | 0.034 | low |
| Varicose veins | 5 | 80.3 | 0 | 2.26(0.41, 12.59) | 0.353 | 0.485 | low |
| Hemoglobin | 4 | 80.1 | 0.002 | -2.12 (-5.42, 1.19) | 0.209 | 0.218 | low |
| Duration of surgery (continuous) | 11 | 97.6 | 0 | -18.35 (-35.34, -1.35) | 0.034 | 0.222 | |
| Types of anesthesia General | 7 | 73.8 | 0.001 | 1.12 (0.67, 1.88) | 0.673 | 0.465 | |
| Cancer | 4 | 11.1 | 0.337 | 0.94(0.41, 2.14) | 0.876 | 0.603 | |
| Coronary artery disease | 3 | 28.2 | 0.249 | 1.59(0.88, 2.85) | 0.122 | 0.406 | |
| Diabetes mellitus | 10 | 71.0 | 0.000 | 1.20(0.65, 2.20) | 0.559 | 0.682 | |
| Stroke | 2 | 0 | 0.498 | 1.95(1.21, 3.13) | 0.006 | ||
| Major bleeding or transfusion | 4 | 75.6 | 0.006 | 1.92(0.77,4.79) | 0.162 | 0.958 | |
| Intraoperative blood loss (continuous) | 6 | 45.5 | 0.102 | -21.92(-46.26, 2.43) | 0.078 | 0.802 | |
| Prophylaxis Mechanical only | 3 | 0 | 0.640 | 0.65(0.51,0.82) | 0.0001 | 0.932 | |
Fig. 2.
Forest plot of meta-analysis for age > 70 years + Forest plot of hypertension meta-analysis + Forest plot of meta-analysis with body mass index ≥ 25
Fig. 3.
Forest plot of operative time analysis + Forest plot of stroke analysis + Forest plot of meta-analysis with mechanical prevention only
Results of multivariate meta-analysis
The results of multivariate analysis mentioned in the included studies were meta-analyzed and combined. The results showed that hypertension, history of cerebrovascular disease, operation time, type of anesthesia, drug prophylaxis (heparin, fondaparinux, and enoxaparin), diabetes mellitus and ambulation on the second day after operation were not significantly associated with venous thrombosis after HA. Female [OR = 1.49, 95% CI (1.17, 1.89), P = 0.001], age > 70 [OR = 1.64, 95% CI (1.36, 1.98), P = 0.0001], BMI of 25 or more [OR = 1.38, 95% CI (1.12, 1.70), P = 0.003], bone cement prosthesis [OR = 4.93, 95% CI (1.34, 18.13), P = 0.016], history of venous thrombosis [OR = 3.99, 95% CI (2.30, 6.92), P = 0.0001] were risk factors for venous thrombosis after HA, as shown in Table 4.
Table 4.
Multivariate Meta-analysis
| Risk factors | No of study | Heterogeneity | OR (95% CI) | P | Egger | Grade rating | |
|---|---|---|---|---|---|---|---|
| I2 (%) | P | ||||||
| Female | 5 | 0 | 0.854 | 1.49 (1.17, 1.89) | 0.001 | 0.397 | low |
| age>70 | 12 | 99 | 0.000 | 1.64 (1.36, 1.98) | 0.0001 | 0.144 | Moderate |
| Hypertension | 2 | 91.9 | 0.000 | 1.99(0.33,12.11) | 0.454 | low | |
| Cerebrovascular disease | 2 | 90.4 | 0.001 | 3.89 (0.29, 51.39) | 0.303 | low | |
| BMI ≥ 25 | 9 | 84.1 | 0.000 | 1.38 (1.12, 1.70) | 0.003 | 0.199 | Moderate low |
| Duration of surgery | 4 | 25.7 | 0.257 | 0.99(0.98, 1.00) | 0.071 | 0.619 | low |
| Types of anesthesia General | 3 | 96 | 0 | 2.07(0.29,15.01) | 0.470 | 0.887 | low |
| Enoxaparin | 2 | 0 | 0.86 | 0.88(0.62,1.25) | 0.482 | low | |
| Heparin | 2 | 0 | 0.788 | 1.44(0.85,2.44) | 0.176 | low | |
| Diabetes mellitus | 4 | 86.4 | 0 | 1.35(0.48, 3.80) | 0.566 | 0.603 | low |
| ambulation after surgery Day 2 | 2 | 93.8 | 0 | 1.05(0.48, 2.31) | 0.903 | ||
| Cemented prosthesis | 2 | 92.5 | 0 | 4.93(1.34, 18.13) | 0.016 | ||
| History of VTE | 2 | 0 | 0.478 | 3.99(2.30, 6.92) | 0.0001 | ||
Publication bias
Egger’s test was used to evaluate the publication bias of each risk factor. The results (P > 0.05 for each index), regardless of univariate or multivariate analysis, indicated that there was no publication bias, as shown in Tables 3 and 4.
Discussion
This study represents the first meta-analysis to explore risk factors for venous thrombosis following HA. Venous thrombosis results from blood clotting within the venous lumen caused by a myriad of factors. The formation process encompasses inflammation, platelet aggregation, endothelial injury, and the synergistic influence of platelets and various other factors.
This study revealed a significantly higher incidence of venous thrombosis in female patients following HA compared to male patients, as determined by both univariate and multivariate analysis. Consequently, it is recommended that medical staff intensify their focus on female patients to mitigate their risk of venous thrombosis [23]. This study also found that the incidence of venous thrombosis after HA increased in patients older than 70 years of age. The reason may be that older patients often have complex physiological degeneration or organic lesions of multiple organs, which leads to a decrease in the response of the coagulation system [34]. Furthermore, hypertension significantly elevated the risk of venous thrombosis following HA. Patients with hypertension often exhibited increased peripheral vascular resistance, leading to elevated blood flow and vascular pressure. This may disrupt the normal flow velocity, subsequently contributing to the development of venous thrombosis [35]. Patients with a BMI of 25 or higher exhibited an increased incidence of post-HA venous thrombosis. This phenomenon may be attributed to the blood viscosity caused by obesity, which subsequently delays the recovery of the lower limbs and ultimately leads to thrombosis [36]. The duration of the operative procedure appeared to be a significant risk factor for post-HA venous thrombosis. This may be attributed to several factors. Firstly, prolonged periods of immobility and anesthesia can result in complete paralysis of lower limb muscles, leading to loss of normal contraction function, muscle relaxation, and the development of varicose veins. Secondly, an increase in operative time could intensify mechanical damage to the vein wall [37]. Furthermore, the prevalence of venous thrombosis in stroke patients stands at 75%. The high risk of venous thrombosis in stroke patients is attributed to diminished mobility and the absence of the extrusion effect from the lower limb muscle pump. Consequently, blood flow is reduced, leading to blood stagnation. This stagnation activates the endogenous coagulation system, thereby facilitating the development of venous thrombosis [38]. According to the American College of Chest Physicians (ACCP) Guidelines for Antithrombotic Therapy [39] and a review by orthopedic expert Jay R. Lieberman published in The Journal of Bone & Joint Surgery [40], mechanical prevention alone is only effective in patients with an extremely high risk of bleeding or contraindications to anticoagulation. For high-risk patients (such as those of advanced age, high BMI, and previous history of thrombosis), combined drug therapy for preventing venous thrombosis (such as low molecular weight heparin, and warfarin) is required. Mechanical prevention alone is not an independent risk factor, but is affected by patient compliance, device type, and usage type. That is, when not used in a standardized manner, it may indirectly increase the risk due to insufficient protection.
Furthermore, cemented prosthesis releases toxic monomers such as methyl methacrylate, which are subsequently isolated and trapped within the pulmonary circulation. This leads to a state of severe hypercoagulability, resulting in vascular occlusion and organ damage. Consequently, this damage affects vital organs such as the brain, heart, and kidneys, leading to the release of vasoactive substances. It causes hemodynamic imbalance, ultimately leading to the formation of venous thrombosis [41]. A history of venous thrombosis has been identified as an independent risk factor for post-HA venous thrombosis. The risk of venous thrombosis in patients with a history of venous thrombosis is over eight times higher than those without such a history [42].
This study identified several risk factors for venous thrombosis after hip replacement. Accordingly, we propose the following postoperative anticoagulation treatment measures for clinicians. (i) Risk stratification is advisable [43]. Patients with only one risk factor or no risk factor are classified as medium- and low-risk patients, and patients with any two or more risk factors are classified as high-risk patients (female sex, age over 70 years, hypertension, a BMI of 25 or higher, stroke, use of a cemented prosthesis, and a history of venous thrombosis). For low- and medium-risk patients, standard anticoagulation therapy is adopted (10–14 days after surgery, such as DOACs: apixaban 2.5 mg bid); an intermittent pneumatic compression device is started within 48 h after surgery; and early ambulation is encouraged. For high-risk patients, the anticoagulation treatment can be extended to 35 days after surgery, and combined prevention is adopted: mechanical prevention (intermittent pneumatic compression device) combined with drug anticoagulation (rivaroxaban 10 mg/d). (ii) Specific interventions should be adopted for specific risk factors [44, 45].
For old patients (> 70 years old), DOACs with lower bleeding risk are preferred, and monitoring renal function (eGFR) is necessary to adjust medication accordingly (rivaroxaban is contraindicated when eGFR is < 30). If BMI ≥ 25, the dose of low molecular weight heparin is calculated according to body weight (enoxaparin 0.5 mg/kg/d), and early postoperative activity (starting ankle pump exercise 6 h after surgery) is imperative. In terms of bone cement prosthesis or surgery time > 3 h, tranexamic acid should be used prophylactically during surgery to reduce bleeding, and anticoagulation should be started on time at 6–12 h after surgery. For female patients, it is necessary to pay attention to the history of hormone replacement therapy or contraceptive use, and suspend estrogen drugs if necessary. DOACs are contraindicated for pregnant patients, and low molecular weight heparin should be used instead. (iii) Multidisciplinary collaboration and dynamic observation are beneficial [45]. The anesthesiology department selects the anesthesia method. The rehabilitation department develops an early activity plan to optimize the overall management process, and clearly explains to patients the importance of individual risks and medication compliance, according to the ACCP (2021) guidelines [39]. Furthermore, it is recommended to dynamically assess the risk of thrombosis and bleeding, review D-dimer and lower limb venous ultrasound at 1 week, 1 month, 3 months, 6 months, and 1 year after surgery to monitor post-thrombotic syndrome, and perform vascular surgery if necessary. Through the above-mentioned comprehensive measures, the efficacy and safety of anticoagulation can be effectively balanced, and the incidence of postoperative venous thrombosis can be reduced.
This study still has several limitations. First, the number of included articles was small, and few studies performed multivariate analysis, which may introduce selection bias. Second, the included studies used different diagnostic criteria for HA and venous thrombosis, which may account for the large heterogeneity. Third, information on preoperative anticoagulant therapy used in patients due to risk factors for various diseases, and the type and duration of postoperative anti-coagulant used after HA surgery is lacking, which may lead to some bias in the results. Fourth, we excluded the large-scale study by Keller et al. (2020). Although this exclusion avoids the excessive influence of a single study on the results and improves the generalizability of the results, it may also weaken the statistical power of certain risk factors (such as coronary artery disease). Future studies need to further verify such associations with larger-scale, multicenter data.
Conclusions
Based on the available evidence, female sex, age over 70 years, hypertension, a BMI of 25 or higher, duration of operation, stroke, mechanical prevention only, use of a cemented prosthesis, and a history of venous thrombosis are risk factors for venous thrombosis after HA. Clinicians can combine these indicators to early detect, diagnose, and intervene patients with venous thrombosis after HA, so as to improve the quality of life of such patients.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1: Search history
Supplementary Figure 1. Sensitivity analysis for age. Supplementary Figure 2. Sensitivity analysis for body mass index. Supplementary Figure 3. Sensitivity analysis for operation duration
Supplementary file 1. Forest plot for female, age > 70, hypertension, stroke, major bleeding or transfusion of blood, and coronary artery disease before excluding the study by Keller et al
Supplementary file 2. Forest plot for female, age > 70, hypertension, stroke, major bleeding or transfusion of blood and coronary artery disease after excluding the study by Keller et al.
Acknowledgements
Not applicable.
Author contributions
All authors contributed to the study conception and design. Writing - original draft preparation: Wang Huang; Writing - review and editing: Weiwei Hu, BangGuo Lei, and Weichen Huang; Conceptualization: Wang Huang and Weiwei Hu; Methodology: Weiwei Hu; Formal analysis and investigation: Wang Huang; Resources: Bangguo Lei; Funding acquisition: Weichen Huang; Supervision: Weichen Huang, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
This study was supported by National Natural Science Foundation of China (grant number 82360948).
Data availability
The original contributions presented in the study are included in the article and supplementary materials.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Wang Huang, Email: 1581826694@qq.com.
Weichen Huang, Email: 651562565@qq.com.
References
- 1.Talia AJ, Coetzee C, Tirosh O, Tran P. Comparison of outcome measures and complication rates following three different approaches for primary total hip arthroplasty: A pragmatic randomised controlled trial. Trials. 2018;19(1):13. 10.1186/s13063-017-2368-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Higgins BT, Barlow DR, Heagerty NE, Lin TJ. Anterior vs. Posterior approach for total hip arthroplasty, a systematic review and meta-analysis. J Arthroplasty. 2015;30(3):419–34. 10.1016/j.arth.2014.10.020. [DOI] [PubMed] [Google Scholar]
- 3.Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the united States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780–5. 10.2106/jbjs.F.00222. [DOI] [PubMed] [Google Scholar]
- 4.Sidhu VS, Kelly TL, Pratt N, Graves SE, Buchbinder R, Adie S, et al. Effect of aspirin vs Enoxaparin on symptomatic venous thromboembolism in patients undergoing hip or knee arthroplasty: the cristal randomized trial. JAMA. 2022;328(8):719–27. 10.1001/jama.2022.13416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matharu GS, Kunutsor SK, Judge A, Blom AW, Whitehouse MR. Clinical effectiveness and safety of aspirin for venous thromboembolism prophylaxis after total hip and knee replacement: A systematic review and meta-analysis of randomized clinical trials. JAMA Intern Med. 2020;180(3):376–84. 10.1001/jamainternmed.2019.6108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tritschler T, Kraaijpoel N, Le Gal G, Wells PS. Venous thromboembolism: advances in diagnosis and treatment. JAMA. 2018;320(15):1583–94. 10.1001/jama.2018.14346. [DOI] [PubMed] [Google Scholar]
- 7.Thomas O, Lybeck E, Strandberg K, Tynngård N, Schött U. Monitoring low molecular weight heparins at therapeutic levels: Dose-responses of, and correlations and differences between Aptt, anti-factor Xa and thrombin generation assays. PLoS ONE. 2015;10(1):e0116835. 10.1371/journal.pone.0116835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herrera S, Comerota AJ. Embolization during treatment of deep venous thrombosis: incidence, importance, and prevention. Tech Vasc Interv Radiol. 2011;14(2):58–64. 10.1053/j.tvir.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 9.Wakabayashi H, Hasegawa M, Niimi R, Sudo A. Clinical analysis of preoperative deep vein thrombosis risk factors in patients undergoing total hip arthroplasty. Thromb Res. 2015;136(5):855–8. 10.1016/j.thromres.2015.06.021. [DOI] [PubMed] [Google Scholar]
- 10.Colwell CW. The accp guidelines for thromboprophylaxis in total hip and knee arthroplasty. Orthopedics. 2009;32(12 Suppl):67–73. 10.3928/01477447-20091103-51. [DOI] [PubMed] [Google Scholar]
- 11.Falck-Ytter Y, Francis CW, Johanson NA, Curley C, Dahl OE, Schulman S, et al. Prevention of Vte in orthopedic surgery patients: antithrombotic therapy and prevention of thrombosis, 9th ed: American college of chest physicians evidence-based clinical practice guidelines. Chest. 2012;141(2 Suppl):eS278–325. 10.1378/chest.11-2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ye M, Chang N, Liang M, Guo L, Li J, Zhang B. Influential factors of deep venous thrombosis of lower limbs in elderly patients of hip replacement. J Pract Orthop. 2020;26(1):4–7. [Google Scholar]
- 13.Yin Q, Han L, Bian Y, Huang X, Lei Y, Song Y, et al. Interpretation of 2021 asia-pacific venous thromboembolism consensus in knee and hip arthroplasty and hip fracture surgery: Pharmacological venous thromboembolism prophylaxis. Her Med. 2022;41(05):599–602. [Google Scholar]
- 14.Parvizi J, Ceylan HH, Kucukdurmaz F, Merli G, Tuncay I, Beverland D. Venous thromboembolism following hip and knee arthroplasty: the role of aspirin. J Bone Joint Surg Am. 2017;99(11):961–72. 10.2106/jbjs.16.01253. [DOI] [PubMed] [Google Scholar]
- 15.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The Prisma statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7):e1000100. 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roberts KC, Brox WT. Aaos clinical practice guideline: management of hip fractures in the elderly. J Am Acad Orthop Surg. 2015;23(2):138–40. 10.5435/jaaos-d-14-00433. [DOI] [PubMed] [Google Scholar]
- 17.Stang A. Critical evaluation of the newcastle-ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–5. 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 18.Keller K, Hobohm L, Barco S, Schmidtmann I, Münzel T, Engelhardt M, et al. Venous thromboembolism in patients hospitalized for hip joint replacement surgery. Thromb Res. 2020;190:1–7. 10.1016/j.thromres.2020.03.019. [DOI] [PubMed] [Google Scholar]
- 19.Deng W, Huo L, Yuan Q, Huang D, Li Q, Tian W. Risk factors for venous thromboembolism in patients with diabetes undergoing joint arthroplasty. BMC Musculoskelet Disord. 2021;22(1):608. 10.1186/s12891-021-04453-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fujita Y, Nakatsuka H, Namba Y, Mitani S, Yoshitake N, Sugimoto E, et al. The incidence of pulmonary embolism and deep vein thrombosis and their predictive risk factors after lower extremity arthroplasty: A retrospective analysis based on diagnosis using multidetector Ct. J Anesth. 2015;29(2):235–41. 10.1007/s00540-014-1891-x. [DOI] [PubMed] [Google Scholar]
- 21.Howard TA, Judd CS, Snowden GT, Lambert RJ, Clement ND. Incidence and risk factors associated with venous thromboembolism following primary total hip arthroplasty in low-risk patients when using aspirin for prophylaxis. Hip Int. 2022;32(5):562–7. 10.1177/1120700021994530. [DOI] [PubMed] [Google Scholar]
- 22.Jiang T, Song K, Yao Y, Pan P, Jiang Q. Perioperative allogenic blood transfusion increases the incidence of postoperative deep vein thrombosis in total knee and hip arthroplasty. J Orthop Surg Res. 2019;14(1):235. 10.1186/s13018-019-1270-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Migita K, Bito S, Nakamura M, Miyata S, Saito M, Kakizaki H, et al. Venous thromboembolism after total joint arthroplasty: results from a Japanese multicenter cohort study. Arthritis Res Ther. 2014;16(4):R154. 10.1186/ar4616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shimoyama Y, Sawai T, Tatsumi S, Nakahira J, Oka M, Nakajima M, et al. Perioperative risk factors for deep vein thrombosis after total hip arthroplasty or total knee arthroplasty. J Clin Anesth. 2012;24(7):531–6. 10.1016/j.jclinane.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 25.Tang J, Zhu W, Mei X, Zhang Z. Plasminogen activator inhibitor-1: A risk factor for deep vein thrombosis after total hip arthroplasty. J Orthop Surg Res. 2018;13(1):8. 10.1186/s13018-018-0716-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.White RH, Gettner S, Newman JM, Trauner KB, Romano PS. Predictors of rehospitalization for symptomatic venous thromboembolism after total hip arthroplasty. N Engl J Med. 2000;343(24):1758–64. 10.1056/nejm200012143432403. [DOI] [PubMed] [Google Scholar]
- 27.Xu H, Zhang S, Xie J, Lei Y, Cao G, Chen G, et al. A nested case-control study on the risk factors of deep vein thrombosis for Chinese after total joint arthroplasty. J Orthop Surg Res. 2019;14(1):188. 10.1186/s13018-019-1231-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu Z, Dai X, Yao Y, Shi D, Chen D, Dai J, et al. Higher levels of serum triglycerides were associated with postoperative deep vein thrombosis after total hip arthroplasty in patients with nontraumatic osteonecrosis of the femoral head. Int J Low Extrem Wounds. 2016;15(1):41–4. 10.1177/1534734615580017. [DOI] [PubMed] [Google Scholar]
- 29.Yao Y, Qiao L, Song K, Xu X, Shi D, Xu Z et al.,. Preoperative evaluation of soleal vein diameter by ultrasound is beneficial for prophylaxis of deep vein thrombosis after total knee or hip arthroplasty. Biomed Res Int. 2018; 2018:3417648.10.1155/2018/3417648. [DOI] [PMC free article] [PubMed]
- 30.Yu X, Wu Y, Ning R. The deep vein thrombosis of lower limb after total hip arthroplasty: what should we care. BMC Musculoskelet Disord. 2021;22(1):547. 10.1186/s12891-021-04417-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yukizawa Y, Inaba Y, Kobayashi N, Kubota S, Saito T. Current risk factors for asymptomatic venous thromboembolism in patients undergoing total hip arthroplasty. Mod Rheumatol. 2019;29(5):874–9. 10.1080/14397595.2018.1524959. [DOI] [PubMed] [Google Scholar]
- 32.Zeng Y, Si H, Wu Y, Yang J, Zhou Z, Kang P, et al. The incidence of symptomatic in-hospital Vtes in Asian patients undergoing joint arthroplasty was low: A prospective, multicenter, 17,660-patient-enrolled cohort study. Knee Surg Sports Traumatol Arthrosc. 2019;27(4):1075–82. 10.1007/s00167-018-5253-3. [DOI] [PubMed] [Google Scholar]
- 33.Zhang S, Xie X, Yao Y, Wei L. Four risk factors of deep venous thrombosis in lower limbs after total hip arthroplasty. Chin J Tissue Eng Res. 2015;19(13):1969–73. [Google Scholar]
- 34.Shahi A, Bradbury TL, Guild GN 3rd, Saleh UH, Ghanem E, Oliashirazi A. What are the incidence and risk factors of in-hospital mortality after venous thromboembolism events in total hip and knee arthroplasty patients? Arthroplast Today. 2018;4(3):343–7. 10.1016/j.artd.2018.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sheng J. Hemorheologic correlation between primary hypertension and hyperviscosity, hypercoagulation and hyperlipidemia. Chin J Hemorheol. 2003;13(3):262–3. [Google Scholar]
- 36.Mantilla CB, Horlocker TT, Schroeder DR, Berry DJ, Brown DL. Risk factors for clinically relevant pulmonary embolism and deep venous thrombosis in patients undergoing primary hip or knee arthroplasty. Anesthesiology. 2003;99(3):552–60. 10.1097/00000542-200309000-00009. [DOI] [PubMed] [Google Scholar]
- 37.Huang J, Liu L, Cai F, Chen Q, Xia S, Liao Q. A meta-analysis on clinical outcomes of simultaneous operation versus staged operation of bilateral total hip arthroplasty in Chinese. Orthop J China. 2020;28(17):1583–7. [Google Scholar]
- 38.Bembenek J, Karlinski M, Kobayashi A, Czlonkowska A. Early stroke-related deep venous thrombosis: risk factors and influence on outcome. J Thromb Thrombolysis. 2011;32(1):96–102. 10.1007/s11239-010-0548-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stevens SM, Woller SC, Kreuziger LB, Bounameaux H, Doerschug K, Geersing GJ, et al. Antithrombotic therapy for Vte disease: second update of the chest guideline and expert panel report. Chest. 2021;160(6):e545–608. 10.1016/j.chest.2021.07.055. [DOI] [PubMed] [Google Scholar]
- 40.Lieberman JR, Pensak MJ. Prevention of venous thromboembolic disease after total hip and knee arthroplasty. J Bone Joint Surg Am. 2013;95(19):1801–11. 10.2106/jbjs.L.01328. [DOI] [PubMed] [Google Scholar]
- 41.Dahl OE, Pripp AH, Jaradeh M, Fareed J. The bone cement hypercoagulation syndrome: pathophysiology, mortality, and prevention. Clin Appl Thromb Hemost. 2023;29. 10.1177/10760296231198036. [DOI] [PMC free article] [PubMed]
- 42.Samama MM. An epidemiologic study of risk factors for deep vein thrombosis in medical outpatients: the Sirius study. Arch Intern Med. 2000;160(22):3415–20. 10.1001/archinte.160.22.3415. [DOI] [PubMed] [Google Scholar]
- 43.Saha P, Black S, Breen K, Patel A, Modarai B, Smith A. Contemporary management of acute and chronic deep venous thrombosis. Br Med Bull. 2016;117(1):107–20. 10.1093/bmb/ldw006. [DOI] [PubMed] [Google Scholar]
- 44.Migliorini F, Maffulli N, Velaj E, Bell A, Kämmer D, Hildebrand F, et al. Antithrombotic prophylaxis following total hip arthroplasty: A level i bayesian network meta-analysis. J Orthop Traumatol. 2024;25(1):1. 10.1186/s10195-023-00742-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chinese Orthopaedic Association. Guidelines for prevention of venous thromboembolism after major orthopedic surgery in China. Chin J Orthop. 2016;265–71. 10.3760/cma.j.issn.0253-2352.2016.02.001.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material 1: Search history
Supplementary Figure 1. Sensitivity analysis for age. Supplementary Figure 2. Sensitivity analysis for body mass index. Supplementary Figure 3. Sensitivity analysis for operation duration
Supplementary file 1. Forest plot for female, age > 70, hypertension, stroke, major bleeding or transfusion of blood, and coronary artery disease before excluding the study by Keller et al
Supplementary file 2. Forest plot for female, age > 70, hypertension, stroke, major bleeding or transfusion of blood and coronary artery disease after excluding the study by Keller et al.
Data Availability Statement
The original contributions presented in the study are included in the article and supplementary materials.