Soluble CD14, a marker of monocyte activation and independent predictor of mortality in HIV disease, is reduced by rosuvastatin treatment. Monocyte tissue factor expression in HIV-infected subjects is reduced by rosuvastatin treatment, potentially reducing thrombotic risk.
Keywords: HIV-1, monocytes, tissue factor, rosuvastatin
Abstract
Background. Statins, or 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors, have anti-inflammatory effects that are independent of their lipid-lowering properties. Despite suppressive antiretroviral therapy (ART), elevated levels of immune activation and inflammation often persist.
Methods. The Stopping Atherosclerosis and Treating Unhealthy Bone With Rosuvastatin in HIV (SATURN-HIV) trial is a randomized, double-blind, placebo-controlled study, designed to investigate the effects of rosuvastatin (10 mg/daily) on markers of cardiovascular disease risk in ART-treated human immunodeficiency virus (HIV)–infected subjects. A preplanned analysis was to assess changes in markers of immune activation at week 24. Subjects with low-density lipoprotein cholesterol <130 mg/dL and heightened immune activation (%CD8+CD38+HLA-DR+ ≥19%, or plasma high-sensitivity C-reactive protein ≥2 mg/L) were randomized to receive rosuvastatin or placebo. We measured plasma (soluble CD14 and CD163) and cellular markers of monocyte activation (proportions of monocyte subsets and tissue factor expression) and T-cell activation (expression of CD38, HLA-DR, and PD1).
Results. After 24 weeks of rosuvastatin, we found significant decreases in plasma levels of soluble CD14 (−13.4% vs 1.2%, P = .002) and in proportions of tissue factor–positive patrolling (CD14DimCD16+) monocytes (−38.8% vs −11.9%, P = .04) in rosuvastatin-treated vs placebo-treated subjects. These findings were independent of the lipid-lowering effect and the use of protease inhibitors. Rosuvastatin did not lead to any changes in levels of T-cell activation.
Conclusions. Rosuvastatin treatment effectively lowered markers of monocyte activation in HIV-infected subjects on antiretroviral therapy.
Clinical Trials Registration NCT01218802.
Inflammation and immune activation may contribute to the progression of cardiovascular disease (CVD) in the general population [1, 2]. Patients infected with the human immunodeficiency virus (HIV) appear to have a greater risk for CVD than uninfected controls [3–6], and within HIV-infected subjects, markers of inflammation, immune activation, and coagulation are associated with mortality, HIV disease progression, vascular disease, and diabetes [7–11]. The drivers of increased inflammation and cardiovascular risk in HIV disease remain unclear, but have been attributed to coinfections with agents such as cytomegalovirus [12], to persistence of HIV replication in sanctuary sites [13], by exposure to bioactive lipids [14–16], to homeostatic cytokines [17], and to microbial elements translocated through a damaged gut [18].
Activated monocytes may contribute to inflammation and cardiovascular disease [19]. Three monocyte subsets can be identified based on CD14 and CD16 expression [20–23]. Traditional (CD14+CD16−) monocytes engulf and present antigen. Patrolling (CD14DimCD16+) monocytes home to the vascular endothelium and recognize viral products; inflammatory (CD14+CD16+) monocytes produce high levels of inflammatory cytokines in response to bacterial products [22]. Proportions of monocytes from HIV-infected donors, and uninfected donors who have recently had an acute coronary event, are enriched for patrolling and inflammatory monocytes, and these cells have a procoagulant phenotype [24]. Soluble CD14 (sCD14), a marker of monocyte activation, is an independent predictor of mortality in HIV-infected subjects [9] and has been linked to faster vascular disease progression, as measured by carotid intima-media thickness [25]. Similarly, the macrophage activation marker soluble CD163 (sCD163) is associated with noncalcified plaques in the coronary arteries [26] and with arterial inflammation in HIV-infected subjects [27]. These markers provide a link between myeloid cell activation and cardiovascular risk in HIV disease.
Identifying successful therapies that would reduce chronic immune activation in treated HIV disease is an ongoing research priority. Statins, or 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors, have anti-inflammatory effects beyond those related to cholesterol lowering [28, 29].
Several statin studies have been performed in HIV-infected subjects, but most were aimed at the effects of statins on lipid levels and on HIV viremia [30–34]. Recently, administration of high-dose atorvastatin (80 mg) modestly reduced the proportion of HLA-DR–expressing CD8+ T cells in HIV-infected subjects who were not receiving antiretroviral therapy (ART) [35]. In our current study, the Stopping Atherosclerosis and Treating Unhealthy Bone With Rosuvastatin in HIV (SATURN-HIV) trial, HIV-infected subjects who were undergoing successful ART and had normal low-density lipoprotein (LDL) cholesterol levels, but elevated levels of inflammation and immune activation, were randomized to receive rosuvastatin (10 mg daily) or placebo. We present the results of a prespecified secondary analysis aimed at assessing the effects of statin administration on markers of immune activation and inflammation, including plasma levels of monocyte/macrophage activation markers (sCD14 and sCD163), and proportions of activated T cells and monocytes.
MATERIALS AND METHODS
Study Design
SATURN-HIV is a randomized, double-blind, placebo-controlled study designed to measure the effect of rosuvastatin on markers of cardiovascular risk, skeletal health, and immune activation in HIV disease (clinicaltrials.gov identifier: NCT01218802). The study was approved by the Institutional Review Board of University Hospitals Case Medical Center (Cleveland, Ohio), and all subjects signed a written consent prior to enrollment. Randomization was conducted by the Case investigational pharmacist as 1:1 to active rosuvastatin 10 mg daily vs matching placebo. Randomization was stratified by protease inhibitor (PI) use. Study drugs (active and placebo) were provided by AstraZeneca.
All subjects were ≥18 years of age, without known coronary disease or diabetes, and on stable ART for at least 3 months and cumulative ART duration of at least 6 months, with HIV type 1 (HIV-1) RNA <1000 copies/mL and fasting LDL cholesterol (LDL-C) level of ≤130 mg/dL and fasting triglyceride level of ≤500 mg/dL. Subjects were excluded if they had a history of myocardial infarction, were pregnant or lactating, or had an active or controlled inflammatory condition. Additional entry criteria included evidence of either heightened T-cell activation, identified as the proportion of CD8+ T cells that expressed CD38+HLA-DR+ ≥19%, or levels of high-sensitivity C-reactive protein (hs-CRP) ≥2 mg/L. The T-cell activation cutoff (>19%) was determined based on our prior data linking this level of immune activation to higher risk of immunologic failure and to the fact that 19% represented the approximately 75% of “normal” level of healthy HIV-uninfected subjects [36]. The CRP cutoff was selected based on criteria established in the JUPITER statin trial [29].
Study Evaluations
At the initial screening visit, self-reported demographics and medical history were obtained along with a targeted physical exam including height and weight measurements. Blood was drawn after a 12-hour fast for lipoproteins, and hs-CRP. The percentage of CD8+ T-cell activation was determined as described below. If enrollment criteria were met, subjects returned within 30 days for entry evaluations. At entry, a fasting blood draw was obtained for a lipid profile and for measurement of soluble markers of immune activation. HIV-1 RNA levels and CD4+ cell counts were obtained as part of routine care.
Blood/Sample Preparation
Whole-blood samples were collected into ethylenediaminetetraacetic acid–containing tubes. Peripheral blood mononuclear cell (PBMC) samples were separated by centrifugation with Ficoll-Hypaque and were cryopreserved until analyzed in batches. Plasma was isolated by centrifugation for 10 minutes at 400g and was frozen at −80°C until thawed once and analyzed in batches.
Flow Cytometry
Monocyte Subset Activation
Whole-blood (300 µL) samples were incubated for 15 minutes on ice with FACS Lyse buffer (BD Biosciences, San Diego, California) and were then washed in buffer (phosphate-buffered saline with 1% bovine serum albumin and 0.1% sodium azide). Cells were then stained for 30 minutes in the dark, on ice, and then washed in buffer and fixed in 1% paraformaldehyde. Monocyte subsets were identified by size, granularity, and by expression of CD14 and CD16 [24]. Isotype gating was used to identify positive expression of surface markers. Cell surface molecule expression was monitored by staining cells with fluorochrome-labeled antibodies: anti–tissue factor (TF; fluorescein isothiocyanate [FITC], American Diagnostica, Stamford, Connecticut), anti-CD14 (Pacific Blue), anti-CD16 (phycoerythrin [PE], both from BD Pharmingen, San Diego, California).
T-Cell Activation
The proportion of activated T cells required for patient screening was determined by analyses of freshly collected whole-blood samples, processed as above. T cells were identified by size and granularity and by positive expression of CD3 and CD4 or CD8. T-cell activation was measured using anti-CD38 (PE), anti–HLA-DR (FITC), anti-CD3 (peridinin chlorophyll protein complex) anti-CD8 (allophycocyanin-cy7), anti-CD4 (allophycocyanin, all from BD Biosciences).
Assessment of T-cell activation for the entry and 24-week timepoints was performed by comparing expression of surface markers on cryopreserved PBMC samples from each patient. Samples were thawed and analyzed in batches. In addition to the T-cell markers described above, analysis of frozen PBMC samples also included a stain for cell viability (Live/Dead Violet, Pacific Blue) and an additional activation marker, PD-1 (PE-Cy7, BD Pharmingen).
Monocytes were analyzed in real time using a Miltenyi MACS Quant flow cytometer (MiltenyiBiotec, BergischGladbach, Germany). MACS Quantify software (version 2.21031.1, MiltenyiBiotec) was used to analyze the data. Initial assessment of T-cell activation for screening/enrollment was performed using an LSR II flow cytometer (Becton-Dickinson, San Jose, California) and FACSDiva software version 6.1.1 (BD Biosciences). Longitudinal T-lymphocyte activation was analyzed on the Quant.
Plasma samples were collected at baseline and week 24. Levels of sCD14 and sCD163 were measured using Quantikine enzyme-linked immunosorbent assay kits (R&D Systems, Minneapolis, Minnesota).
Statistical Methods
This was a prespecified, preplanned analysis to assess changes from baseline to 24 weeks in markers of lymphocyte and monocyte activation.
All analyses were initially performed using intent-to-treat principles based on randomized treatment assignment that used all available data. Modifications to randomized treatment and missing values were ignored. As-treated analyses did not differ from intent-to-treat analyses; therefore, only the former data are presented.
Demographics, clinical characteristics, fasting metabolic parameters, and inflammatory and coagulation markers are described by study group. Continuous measures are described by medians and interquartile ranges, and nominal variables are described with frequencies and percentages.
Nominal variables were compared using χ2 analysis or Fisher exact test. Continuous measures were tested for normality. For between-group comparisons (at baseline and changes from baseline to 24 weeks), normally distributed variables were compared using t tests, and nonnormally-distributed variables were compared using Wilcoxon rank-sum tests. For within-group changes from baseline to 24 weeks, normally distributed variables were compared with a paired t test, and nonnormally-distributed variables were compared with Wilcoxon signed-rank test.
RESULTS
Baseline Characteristics
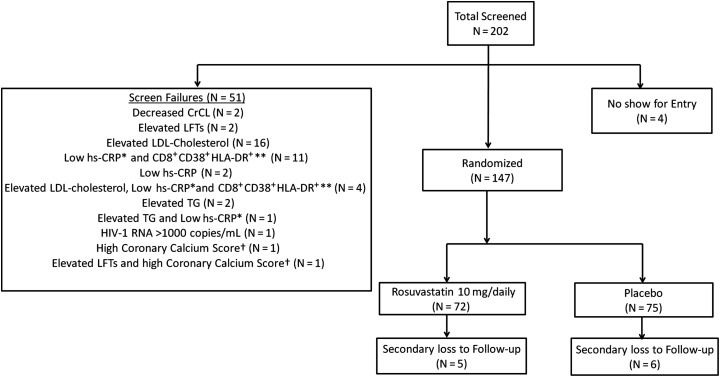
Two hundred two patients were screened for enrollment; 55 patients failed screening, resulting in enrollment of 147 patients between March 2011 and August 2012 in the SATURN-HIV study (n = 72 in the rosuvastatin arm, n = 75 in the placebo arm, Figure 1). Demographic information and baseline characteristics are displayed in Table 1; indices were similar between groups. Overall, the median age of the patients was 47 years; 78% were male, and 70% were African American, 29% were white, and 1% was Hispanic. Baseline immune activation markers were also similar between groups, except for the proportion of CD14DimCD16+ monocytes that expressed TF (statin arm = 21.8%, placebo arm = 18.9%, P = .047; Table 1); this difference was controlled for in the week 24 analysis. There was no difference in the number of subjects from each group on antihypertensive medication (statin: n = 20 vs placebo: n = 18, P = .60) and only 1 subject was on a diabetes medication at baseline. Seven subjects had active hepatitis B (statin: n = 3 vs placebo: n = 4, P = .74), and 12 subjects had active hepatitis C (statin: n = 5 vs placebo: n = 7, P = .60).
Figure 1.
Patient enrollment flowchart. Two hundred two patients were screened for enrollment; 55 patients failed screening, resulting in 147 patients being enrolled (n = 72 in the rosuvastatin arm, n = 75 in the placebo arm). *High-sensitivity C-reactive protein level <2 mg/L. **CD8+ T-cell expression of CD38 and HLA-DR antigens <19%. †Patient found to have very high coronary calcium score by computed tomography on same day as screening, deemed unethical for patient to be potentially randomized to placebo. Abbreviations: CrCL, creatinine clearance; HIV-1, human immunodeficiency virus type 1; hs-CRP, high-sensitivity C-reactive protein; LDL, low-density lipoprotein; LFTs, liver function tests; TG, triglycerides.
Table 1.
Baseline Demographic, Virologic, and Immunologic Characteristics
| Characteristic | Statin (n = 72) | Placebo (n = 75) |
|---|---|---|
| Age, y | 45.6 (41.1–51.4) | 46.9 (39.2–53.6) |
| Male sex | 81% | 76% |
| African American race | 71% | 69% |
| Body mass index, kg/m2 | 26.6 (23.4–30.0) | 27.2 (23.5–30.5) |
| Current smoking | 60% | 72% |
| Active hepatitis B | 4% | 5% |
| Active hepatitis C | 7% | 9% |
| Framingham Risk Score | 3 (1–7.5) | 4 (1–7) |
| CD4+ count, cells/µL | 607.5 (439.5–847.5) | 627.0 (398.0–853.0) |
| Nadir CD4+ count, cells/µL | 172.5 (83.5–312.0) | 189.5 (89.0–281.0) |
| HIV-1 RNA <50 copies/mL | 78% | 77% |
| Duration of HIV+ diagnosis, mo | 133 (75–199) | 145 (73–232) |
| Duration of ART, mo | 63 (37–119) | 71 (39–116) |
| On PI at entry | 50% | 48% |
| Duration of PI, mo | 47 (13–106) | 39 (2–80) |
| CD8+CD38+HLA-DR+, % | 13.3 (9.0–19.1) | 11.5 (8.0–16.5) |
| CD4+CD38+HLA-DR+, % | 5.3 (3.7–6.8) | 5.1 (3.5–6.3) |
| Soluble CD14, ng/mL | 2178 (1783–2497) | 2138 (1611–2455) |
| Soluble CD163, ng/mL | 645 (501–822) | 651 (475–901) |
| CD14+CD16+ monocytes, % | 22.9 (18.1–34.0) | 23.4 (18.6–35.9) |
| CD14DimCD16+ monocytes, % | 12.7 (8.8–15.5) | 10.0 (7.5–14.3) |
| CD14+CD16+TF+, % | 13.4 (7.9–17.4) | 10.9 (7.7–15.9) |
| CD14DimCD16+TF+, % | 21.8 (15.6–29.6) | 18.9 (12.6–25.3) |
Data are reported as median (interquartile range) unless otherwise indicated. The median percentages of CD8+CD38+HLA-DR+ cells reported in this table (on study) are based on results from thawed peripheral blood mononuclear cell samples; percentages used for screening were generated from fresh whole-blood samples. Values generated from thawed samples are notoriously lower than those from fresh samples; this explains why the median values displayed here are less than the screen failure cutoff of 19%.
Abbreviations: ART, antiretroviral therapy; HIV, human immunodeficiency virus; PI, protease inhibitor; TF, tissue factor.
Subject Disposition and Safety Data
Eleven subjects (5 statin; 6 placebo) withdrew prior to the week 24 analysis: 10 were secondary to loss to follow-up. One subject withdrew due to a potential adverse event (on day 4 of study, grade 2 myalgias leading subject to refuse to continue in study or present for follow-up). One additional subject in the statin group stopped treatment at week 5 due to hospitalization for hydration secondary to grade 3 myalgias without rhabdomyolysis or renal compromise, but continued to be followed on study, off the study drug. All myalgias resolved soon after study drugs were discontinued. Two subjects changed ART regimens between baseline and 24 weeks: 1 was started on abacavir in place of didanosine, and 1 stopped lamivudine/zidovudine and started emtricitabine/tenofovir and maraviroc. One subject stopped ART and had an HIV-1 RNA level of >12 000 copies/mL at 24 weeks, but there was no statistical difference in the number of subjects with an undetectable HIV-1 RNA level between baseline and 24 weeks in either group (83% and 84% in statin and placebo groups, respectively). The patient flowchart is provided as Figure 1. Neither HIV-1 RNA levels, nor CD4+ T-cell counts, or their changes, were significantly different between the groups at 24 weeks.
Changes in Fasting Lipid Levels
Lipid profiles in the rosuvastatin group changed by week 24, with significant increases in high-density lipoprotein (HDL; relative increase from baseline = 7.5%, P = .0004) and decreases in LDL (−25.3%, P < .0001), and triglyceride (−2.28%, P < .039) levels. Patients receiving placebo did not have significant changes in these indices (HDL = 5.12%, P = .19; LDL = 6.86%, P = .105; and triglycerides = 4.52%, P = .66).
Changes in Soluble Markers of Monocyte Activation
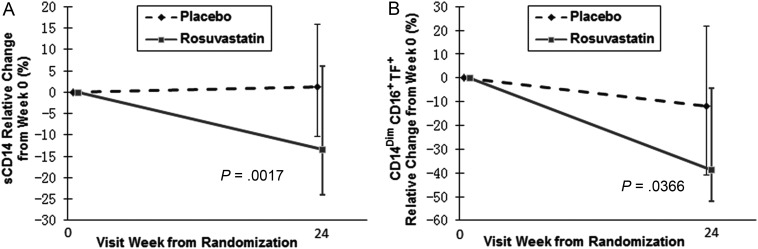
There was no significant difference in week 24 plasma levels of sCD163 between groups (Table 2); rosuvastatin administration reduced levels of sCD14 by 13.4%, relative to baseline levels, while subjects receiving placebo had a 1.2% increase in sCD14 levels (P = .0017; Figure 2A). Rosuvastatin-mediated decreases in sCD14 were weakly associated with decreases in LDL-C, but this was not statistically significant (r = 0.16, P = .067).
Table 2.
Summary Data of Plasma and Cellular Markers of Activation/Inflammation
| Activation Markers | Statin (n = 72) |
Placebo (n = 75) |
P Value Between 2 Groups |
||
|---|---|---|---|---|---|
| Median (Q1, Q3) | Within-Group P Value |
Median (Q1, Q3) | Within-Group P Value |
||
| Lymphocyte activation | |||||
| CD8+CD38+HLA-DR+, % | −5.8% (−21.5,12.0) | .2791 | −4.8% (−19.1, 19.9) | .5992 | .51 |
| CD8+CD38+, % | −0.7% (−9.2, 6.5) | .9578 | 2.6% (−9.1, 10.5) | .3600 | .54 |
| CD8+CD38+HLA-DR+PD1+, % | −2.4% (−26.7, 6.2) | .8896 | 0.9% (−24.7, 30.0) | .3980 | .50 |
| CD4+CD38+HLA-DR+, % | −8.9% (−19.8, 9.5) | .1287 | −4.2% (−21.8, 10.9) | .1542 | .93 |
| CD4+CD38+, % | −0.2% (−4.7, 5.51) | .7410 | −0.8% (−6.6, 6.2) | .6415 | .51 |
| CD4+CD38+HLA-DR+PD1+, % | −10.1% (−20.8, 24.6) | .5559 | −2.8% (−24.1, 32.0) | .9326 | .83 |
| Monocyte activation | |||||
| Soluble CD14, ng/mL | −13.4% (−24.1, −6.0) | .0009 | 1.2% (−10.5, 16.0) | .2116 | .002 |
| Soluble CD163, ng/mL | −3.3% (−19.3, 7.5) | .2645 | −10.5% (−26.4, 15.6) | .1295 | .16 |
| CD14+CD16+ monocytes, % | −18.3% (−37.0,33.7) | .4855 | −5.4% (−31.7, 22.9) | .4924 | .60 |
| CD14DimCD16+ monocytes, % | −0.1% (−31.1,44.0) | .4768 | 7.1% (−32.2, 37.8) | .2766 | .72 |
| CD14+CD16+TF+, % | −38.6% (−55.5, 3.1) | .2022 | −14.4% (−47.6, 20.9) | .2257 | .14 |
| CD14DimCD16+TF+, % | −38.8% (−51.9, −4.2) | .0016 | −11.9% (−41.0, 21.7) | .3119 | .04 |
Plasma samples were thawed and levels of soluble CD14 and soluble CD163 were measured by enzyme-linked immunosorbent assay. Monocyte subsets were identified in fresh whole-blood samples using size and granularity, and by expression of CD14 and CD16. Monocyte tissue factor expression was measured concurrently. Measurement of T-cell activation was performed by comparing expression of surface markers (CD38, HLA-DR, and PD-1) on cryopreserved peripheral blood mononuclear cell samples from baseline and week 24 for each patient. Samples were thawed and analyzed in batches. Median values and interquartile ranges are reported.
Figure 2.
Statin treatment reduces levels of monocyte activation. Plasma samples were thawed and levels of soluble CD14 (sCD14) were measured by enzyme-linked immunosorbent assay. A, Patients receiving statin treatment had a relative reduction in sCD14 levels from baseline (13.4%), whereas patients receiving placebo had an increase in sCD14 levels (1.2%), and this difference was significant between groups (P = .0017). B, Tissue factor (TF) expression on patrolling (CD14DimCD16+) monocytes was also reduced by statin treatment (−38.8%), and this change differed significantly from the change measured in the placebo arm (11.9%, P = .04).
Changes in Cellular Markers of Monocyte and Lymphocyte Activation
We also monitored changes in several cellular indices of immune activation. We did not detect any changes in the proportional representation of monocyte subsets between the rosuvastatin and placebo groups. The proportion of total monocytes that expressed TF decreased significantly within the statin arm (−41%, P = .04), but not within the placebo arm (−11%, P = .69); the difference between treatment groups did not reach significance (P = .1). Among the individual monocyte subsets, subjects receiving rosuvastatin had a greater reduction in the proportion of TF+CD14DimCD16+ (patrolling) monocytes compared to the reduction seen in subjects receiving placebo (−38.8% vs −11.9%, relative to baseline levels, P = .04; Figure 2B). This reduction in the proportion of TF+ patrolling monocytes was not correlated with the observed decrease in plasma LDL-C levels (r = 0.038, P = .7693). As shown in Table 2, statin therapy did not lead to changes in T-cell activation markers (CD38, HLA-DR, PD1) on either CD4+ or CD8+ T cells.
Effect of Statin Treatment in the Subset of Subjects Receiving PIs
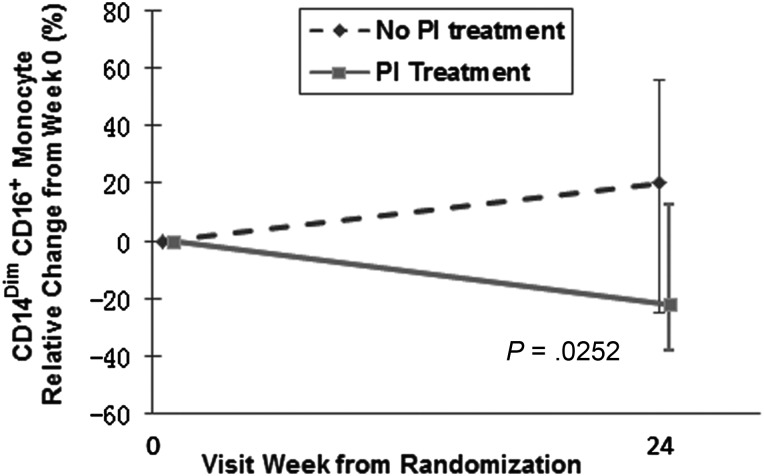
Among the 72 subjects in the rosuvastatin arm, 36 were receiving a PI-based regimen. Rosuvastatin-treated subjects receiving a PI-based ART regimen had a statistically significant decrease in the proportion of CD14DimCD16+ cells compared to the change measured in statin-treated subjects not receiving PI-based ART (−22% vs 20%, relative to baseline, P = .0252; Figure 3A). When comparing the changes in the proportions of these cells between the active and the placebo arms among subjects receiving a PI-based regimen, there was a trend toward significance (statin: −21.7%, placebo: 13.3%, P = .07), suggesting that the decrease in the proportion of these cells was related to statin treatment and not PI treatment. There were no statistically significant differences in any other marker of immune activation between rosuvastatin-treated subjects receiving a PI-based regimen at entry vs those not receiving a PI. Further analyses comparing changes between treatment arms among subjects receiving PI-based ART revealed that there was a trend toward significant differences in sCD14 levels (−10.7% vs 1.4%, P = .08), and the proportion of inflammatory monocytes that express TF (−40.2% vs −6.8%, P = .09); there were significant differences in changes in the proportions of TF+ patrolling monocytes (−39.6% vs 8.5%, P = .03) and the proportions of TF+ total monocytes (−45.3% vs −3.5%, P = .05) between subjects receiving rosuvastatin and placebo, respectively. Among subjects not receiving PI-based ART, there was also a significant difference in the change in sCD14 levels (−14.8% vs −1.4%, P = .009) between treatment arms.
Figure 3.
Rosuvastatin-treated subjects receiving a protease inhibitor (PI)–based antiretroviral therapy regimen had a statistically significant decrease in the proportion of CD14DimCD16+ cells. Monocyte subsets were identified in fresh whole-blood samples using size and granularity, and by expression of CD14 and CD16. Proportions of patrolling (CD14DimCD16+) monocytes were reduced in patients receiving PI-based antiretroviral therapy (ART) (−22%), but not in patients receiving non-PI-based ART (20%, P = .0252).
DISCUSSION
The SATURN-HIV study is a double-blind, randomized, placebo-controlled, clinical trial with a prespecified secondary analysis aimed at assessing the effects of 10 mg/day rosuvastatin treatment on markers of immune activation in HIV-infected subjects on stable ART with low or undetectable HIV-1 RNA levels. We report that rosuvastatin treatment, when compared to placebo, reduces significantly the proportion of circulating TF+ patrolling (CD14DimCD16+) monocytes. Tissue factor can initiate the extrinsic clotting pathway [37]. Decreasing TF expression on patrolling monocytes may have particular importance to CVD risk, as patrolling monocytes home in to the vascular endothelium [22] where they may initiate clot formation. We have previously reported that TF+ monocytes are directly related to plasma D-dimer levels [38], potentially linking monocyte activation to coagulation in HIV-infected subjects. Also, TF expression is increased on circulating monocytes in HIV-uninfected persons with recent acute coronary events [24]. Statin-induced reduction of TF expression is not completely novel; Owens et al reported that simvastatin treatment reduces TF expression on monocytes in hypercholesterolemic mice and monkeys [39]. Simvastatin treatment decreases Toll-like receptor 4 expression on monocytes and alters signaling downstream of this receptor [40], including upregulation of the Kruppel-like factor 2 transcription factor [41], a negative regulator of TF expression [42]. In our study, the reduction in TF expression after rosuvastatin was independent of the LDL-C decrease, supporting the literature demonstrating lipid independent modulation of immune activation by statins [28, 29]. Although we did not see a significant reduction in TF+ monocytes in the traditional (CD14+CD16−) and inflammatory (CD14+CD16+) subsets when comparing the placebo and the statin groups, we found a reduction in TF expression on these monocyte subsets among the subjects in the statin arm; this within-group trend suggests that differences may become apparent by study end.
Previous studies of statin therapy in HIV-infected subjects mainly focused on reduction of viremia, and reported mixed results [30–34]. Recently, high-dose atorvastatin (80 mg) reduced modestly the proportions of HLA-DR+CD4+ and CD8+ T cells in HIV-infected patients who were not receiving ART. We did not find that rosuvastatin treatment reduced T-cell activation, but all subjects in SATURN-HIV are receiving ART, potentially accounting for the difference between studies.
Statin treatment, compared to placebo, reduced significantly levels of plasma sCD14 by 24 weeks. Soluble CD14, a marker of monocyte activation, is an independent predictor of mortality in HIV-infected subjects [9] and a reduction of this magnitude in sCD14 levels may be clinically important, as a 13% decrease in sCD14 was associated with an estimated 21% decrease in non-AIDS morbid events or death, based on risk findings from a study among virologically suppressed subjects [43]. The reduction in sCD14 levels we report with rosuvastatin treatment is considerable. Previously, initiation of nonnucleoside reverse transcriptase inhibitor–based ART did not lower sCD14 levels; however, among subjects receiving raltegravir-based ART, sCD14 levels decreased by 20% (350 ng/mL) [44], a decrease that is similar to the decrease reported here (−13%, 279.8 ng/mL). Our results suggest that rosuvastatin administration to virally suppressed HIV-infected subjects may have a significant, and potentially clinically important, effect on immune activation. The study is ongoing and will assess whether these changes will be associated with improvement in indices of cardiovascular disease risk in these subjects.
Statin therapy may provide a multitude of clinical benefits. Indeed, the improvement seen in immune activation may at least partially explain the decreases in mortality [45, 46], non-AIDS events [47], and non-Hodgkin lymphomas [48] reported in epidemiologic studies of statin therapy in HIV infection.
There are limitations to this study. We investigated a specific HIV population: those on stable ART with low or undetectable HIV-1 RNA, normal LDL-C, and heightened immune activation at baseline. The generalizability to the HIV population as a whole should be determined in other studies. Also, 24 weeks of therapy may be insufficient to see significant changes in many immune activation markers; an earlier atorvastatin study, however, found significant changes in T-cell activation markers after 18 weeks [35]. Also, the clinical benefit of reducing TF expression on monocyte subsets is not known. Nonetheless, this ongoing study will evaluate the longer-term effects of statin therapy on both immune activation markers and indices of cardiovascular disease risk.
Notes
Author contributions. N. T. F., J. R., and B. C. performed experiments. N. S., D. L., M. M. L., and G. A. M. obtained patient samples. S. M. D. and Y. J. provided statistical support. All authors contributed to data analysis and writing of the manuscript.
Financial support. This work was supported by the National Institutes of Health (R01 NR012642 to G. A. M. and 1K99HL108743 to N. T. F.) and by the Center for AIDS Research, Case Western Reserve University (P30 AI36219). Study drugs and matching placebo were donated by AstraZeneca.
Potential conflicts of interest. G. A. M. serves as a consultant and speaker and has received research funding from Bristol-Myers Squibb, GlaxoSmithKline, Gilead, Merck, and Tibotec. G. A. M. currently chairs a data safety monitoring board for a Pfizer-funded study. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–95. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 2.Hansson GK, Hermansson A. The immune system in atherosclerosis. Nat Immunol. 2011;12:204–12. doi: 10.1038/ni.2001. [DOI] [PubMed] [Google Scholar]
- 3.Hsue PY, Giri K, Erickson S, et al. Clinical features of acute coronary syndromes in patients with human immunodeficiency virus infection. Circulation. 2004;109:316–9. doi: 10.1161/01.CIR.0000114520.38748.AA. [DOI] [PubMed] [Google Scholar]
- 4.Hsue PY, Lo JC, Franklin A, et al. Progression of atherosclerosis as assessed by carotid intima-media thickness in patients with HIV infection. Circulation. 2004;109:1603–8. doi: 10.1161/01.CIR.0000124480.32233.8A. [DOI] [PubMed] [Google Scholar]
- 5.Tabib A, Leroux C, Mornex JF, Loire R. Accelerated coronary atherosclerosis and arteriosclerosis in young human-immunodeficiency-virus-positive patients. Coron Artery Dis. 2000;11:41–6. doi: 10.1097/00019501-200002000-00008. [DOI] [PubMed] [Google Scholar]
- 6.Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab. 2007;92:2506–12. doi: 10.1210/jc.2006-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kalayjian RC, Machekano RN, Rizk N, et al. Pretreatment levels of soluble cellular receptors and interleukin-6 are associated with HIV disease progression in subjects treated with highly active antiretroviral therapy. J Infect Dis. 2010;201:1796–805. doi: 10.1086/652750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuller LH, Tracy R, Belloso W, et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008;5:e203. doi: 10.1371/journal.pmed.0050203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sandler NG, Wand H, Roque A, et al. Plasma levels of soluble CD14 independently predict mortality in HIV infection. J Infect Dis. 2011;203:780–90. doi: 10.1093/infdis/jiq118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown TT, Tassiopoulos K, Bosch RJ, Shikuma C, McComsey GA. Association between systemic inflammation and incident diabetes in HIV-infected patients after initiation of antiretroviral therapy. Diabetes Care. 2010;33:2244–9. doi: 10.2337/dc10-0633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ross AC, Rizk N, O'Riordan MA, et al. Relationship between inflammatory markers, endothelial activation markers, and carotid intima-media thickness in HIV-infected patients receiving antiretroviral therapy. Clin Infect Dis. 2009;49:1119–27. doi: 10.1086/605578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sacre K, Hunt PW, Hsue PY, et al. A role for cytomegalovirus-specific CD4+CX3CR1+ T cells and cytomegalovirus-induced T-cell immunopathology in HIV-associated atherosclerosis. AIDS. 2012;26:805–14. doi: 10.1097/QAD.0b013e328351f780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cory TJ, Schacker TW, Stevenson M, Fletcher CV. Overcoming pharmacologic sanctuaries. Curr Opin HIV AIDS. 2013;8:190–5. doi: 10.1097/COH.0b013e32835fc68a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grinspoon S, Carr A. Cardiovascular risk and body-fat abnormalities in HIV-infected adults. N Engl J Med. 2005;352:48–62. doi: 10.1056/NEJMra041811. [DOI] [PubMed] [Google Scholar]
- 15.Piconi S, Parisotto S, Rizzardini G, et al. Atherosclerosis is associated with multiple pathogenic mechanisms in HIV-infected antiretroviral-naive or treated individuals. AIDS. 2013;27:381–9. doi: 10.1097/QAD.0b013e32835abcc9. [DOI] [PubMed] [Google Scholar]
- 16.Rose H, Hoy J, Woolley I, et al. HIV infection and high density lipoprotein metabolism. Atherosclerosis. 2008;199:79–86. doi: 10.1016/j.atherosclerosis.2007.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fry TJ, Connick E, Falloon J, et al. A potential role for interleukin-7 in T-cell homeostasis. Blood. 2001;97:2983–90. doi: 10.1182/blood.v97.10.2983. [DOI] [PubMed] [Google Scholar]
- 18.Klatt NR, Funderburg NT, Brenchley JM. Microbial translocation, immune activation, and HIV disease. Trends Microbiol. 2013;21:6–13. doi: 10.1016/j.tim.2012.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Woollard KJ, Geissmann F. Monocytes in atherosclerosis: subsets and functions. Nat Rev Cardiol. 2010;7:77–86. doi: 10.1038/nrcardio.2009.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ancuta P, Rao R, Moses A, et al. Fractalkine preferentially mediates arrest and migration of CD16+ monocytes. J Exp Med. 2003;197:1701–7. doi: 10.1084/jem.20022156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ancuta P, Wang J, Gabuzda D. CD16+ monocytes produce IL-6, CCL2, and matrix metalloproteinase-9 upon interaction with CX3CL1-expressing endothelial cells. J Leukoc Biol. 2006;80:1156–64. doi: 10.1189/jlb.0206125. [DOI] [PubMed] [Google Scholar]
- 22.Cros J, Cagnard N, Woollard K, et al. Human CD14dim monocytes patrol and sense nucleic acids and viruses via TLR7 and TLR8 receptors. Immunity. 2010;33:375–86. doi: 10.1016/j.immuni.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ziegler-Heitbrock L. The CD14+ CD16+ blood monocytes: their role in infection and inflammation. J Leukoc Biol. 2007;81:584–92. doi: 10.1189/jlb.0806510. [DOI] [PubMed] [Google Scholar]
- 24.Funderburg NT, Zidar DA, Shive C, et al. Shared monocyte subset phenotypes in HIV-1 infection and in uninfected subjects with acute coronary syndromes. Blood. 2012;120:4599–608. doi: 10.1182/blood-2012-05-433946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kelesidis T, Kendall MA, Yang OO, Hodis HN, Currier JS. Biomarkers of microbial translocation and macrophage activation: association with progression of subclinical atherosclerosis in HIV-1 infection. J Infect Dis. 2012;206:1558–67. doi: 10.1093/infdis/jis545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burdo TH, Lo J, Abbara S, et al. Soluble CD163, a novel marker of activated macrophages, is elevated and associated with noncalcified coronary plaque in HIV-infected patients. J Infect Dis. 2011;204:1227–36. doi: 10.1093/infdis/jir520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Subramanian S, Tawakol A, Burdo TH, et al. Arterial inflammation in patients with HIV. JAMA. 2012;308:379–86. doi: 10.1001/jama.2012.6698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jain MK, Ridker PM. Anti-inflammatory effects of statins: clinical evidence and basic mechanisms. Nat Rev. 2005;4:977–87. doi: 10.1038/nrd1901. [DOI] [PubMed] [Google Scholar]
- 29.Ridker PM, Danielson E, Fonseca FA, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359:2195–207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 30.del Real G, Jimenez-Baranda S, Mira E, et al. Statins inhibit HIV-1 infection by down-regulating Rho activity. J Exp Med. 2004;200:541–7. doi: 10.1084/jem.20040061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sklar PA, Masur H, Grubb JR, et al. Pravastatin does not have a consistent antiviral effect in chronically HIV-infected individuals on antiretroviral therapy. AIDS. 2005;19:1109–11. doi: 10.1097/01.aids.0000174461.31794.50. [DOI] [PubMed] [Google Scholar]
- 32.Waters L, Stebbing J, Jones R, et al. The effect of statins on HIV rebound and blips. J Acquir Immune Defic Syndr. 2005;39:637–8. [PubMed] [Google Scholar]
- 33.Moncunill G, Negredo E, Bosch L, et al. Evaluation of the anti-HIV activity of statins. AIDS. 2005;19:1697–700. doi: 10.1097/01.aids.0000183517.60384.db. [DOI] [PubMed] [Google Scholar]
- 34.Probasco JC, Spudich SS, Critchfield J, et al. Failure of atorvastatin to modulate CSF HIV-1 infection: results of a pilot study. Neurology. 2008;71:521–4. doi: 10.1212/01.wnl.0000325006.84658.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ganesan A, Crum-Cianflone N, Higgins J, et al. High dose atorvastatin decreases cellular markers of immune activation without affecting HIV-1 RNA levels: results of a double-blind randomized placebo controlled clinical trial. J Infect Dis. 2011;203:756–64. doi: 10.1093/infdis/jiq115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lederman MM, Calabrese L, Funderburg NT, et al. Immunologic failure despite suppressive antiretroviral therapy is related to activation and turnover of memory CD4 cells. J Infect Dis. 2011;204:1217–26. doi: 10.1093/infdis/jir507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mackman N. Role of tissue factor in hemostasis and thrombosis. Blood Cells Mol Dis. 2006;36:104–7. doi: 10.1016/j.bcmd.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 38.Funderburg NT, Mayne E, Sieg SF, et al. Increased tissue factor expression on circulating monocytes in chronic HIV infection: relationship to in vivo coagulation and immune activation. Blood. 2010;115:161–7. doi: 10.1182/blood-2009-03-210179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Owens AP, 3rd, Passam FH, Antoniak S, et al. Monocyte tissue factor-dependent activation of coagulation in hypercholesterolemic mice and monkeys is inhibited by simvastatin. J Clin Invest. 2012;122:558–68. doi: 10.1172/JCI58969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Methe H, Kim JO, Kofler S, Nabauer M, Weis M. Statins decrease Toll-like receptor 4 expression and downstream signaling in human CD14+ monocytes. Arterioscler Thromb Vasc Biol. 2005;25:1439–45. doi: 10.1161/01.ATV.0000168410.44722.86. [DOI] [PubMed] [Google Scholar]
- 41.Tuomisto TT, Lumivuori H, Kansanen E, et al. Simvastatin has an anti-inflammatory effect on macrophages via upregulation of an atheroprotective transcription factor, Kruppel-like factor 2. Cardiovasc Res. 2008;78:175–84. doi: 10.1093/cvr/cvn007. [DOI] [PubMed] [Google Scholar]
- 42.Lin Z, Kumar A, SenBanerjee S, et al. Kruppel-like factor 2 (KLF2) regulates endothelial thrombotic function. Circ Res. 2005;96:e48–57. doi: 10.1161/01.RES.0000159707.05637.a1. [DOI] [PubMed] [Google Scholar]
- 43.Tenorio AR, Zheng Y, Bosch RJ, et al. Soluble markers of inflammation and coagulation, but not T-cell activation, predict non-AIDS-defining events during suppressive antiretroviral therapy.. In: Conference on Retroviruses and Opportunistic Infections,; 3–6 March 2013; Atlanta, GA,. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Asmuth DM, Ma ZM, Mann S, et al. Gastrointestinal-associated lymphoid tissue immune reconstitution in a randomized clinical trial of raltegravir versus non-nucleoside reverse transcriptase inhibitor-based regimens. AIDS. 2012;26:1625–34. doi: 10.1097/QAD.0b013e3283546595. [DOI] [PubMed] [Google Scholar]
- 45.Rasmussen LD, Kronborg G, Larsen CS, Pedersen C, Gerstoft J, Obel N. Statin therapy and mortality in HIV-infected individuals: a Danish nationwide population-based cohort study. PLoS One. 2013;8:e52828. doi: 10.1371/journal.pone.0052828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moore RD, Bartlett JG, Gallant JE. Association between use of HMG CoA reductase inhibitors and mortality in HIV-infected patients. PLoS One. 2011;6:e21843. doi: 10.1371/journal.pone.0021843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Drechsler H, Zhang S, Maalouf N, Cutrell J, Tebas P, Bedimo R. Impact of statin exposure on mortality and non-AIDS complications in HIV patients on HAART. Conference on Retroviruses and Opportunistic Infections,; 3–6 March 2013; Atlanta, GA,. [Google Scholar]
- 48.Chao C, Xu L, Abrams DI, et al. HMG-CoA reductase inhibitors (statins) use and risk of non-Hodgkin lymphoma in HIV-positive persons. AIDS. 2011;25:1771–7. doi: 10.1097/QAD.0b013e328349c67a. [DOI] [PMC free article] [PubMed] [Google Scholar]