Abstract
Introduction:
Recent studies have identified subtypes of small cell lung carcinoma (SCLC) defined by RNA expression of ASCL1, NEUROD1, POU2F3 and YAP1 transcriptional regulators. There are only limited data on distribution of these markers at the protein level and associated pathologic characteristics in clinical SCLC samples.
Methods:
Expression of ASCL1, NEUROD1, POU2F3 and YAP1 was analyzed by immunohistochemistry in 174 SCLC patient samples. Subtypes defined by these markers were correlated with histologic characteristics, expression of neuroendocrine markers (synaptophysin, chromogranin A, CD56, INSM1) and other SCLC markers including neuroendocrine phenotype-associated marker DLL3.
Results:
ASCL1 and NEUROD1 expression had the following distribution: ASCL1+/NEUROD1− 41%, ASCL1+/NEUROD1+ 37%, ASCL1−/NEUROD1+ 8% and ASCL1−/NEUROD1− 14%. Based on the relative expression, 69% of cases were ASCL1-dominant and 17% NEUROD1-dominant. POU2F3 was expressed in 7% of SCLC, and was mutually exclusive of ASCL1 and NEUROD1. YAP1 was expressed at low levels, primarily in combined SCLC, and was not exclusive of other subtypes. Both ASCL1-dominant and NEUROD1-dominant subtypes were associated with neuroendocrine markerhigh/DLL3high profile, whereas POU2F3 and other ASCL1/NEUROD1-double-negative tumors were neuroendocrine markerlow/DLL3low.
Conclusions:
This is the first comprehensive immunohistochemical and histopathologic analysis of novel SCLC subtypes in patient samples. We confirm that ASCL1/NEUROD1-double-negative tumors represent a distinct neuroendocrine-low subtype of SCLC which is either uniquely associated with POU2F3 or lacks a known dominant regulator. Expression profiles of these markers appear more heterogeneous in native samples than in experimental models, particularly in regard to high prevalence of ASCL1/NEUROD1 co-expression. These findings may have prognostic and therapeutic implications and warrant further clinical investigation.
Keywords: ASCL1, NEUROD1, POU2F3, YAP1, small cell lung carcinoma, neuroendocrine
INTRODUCTION:
Small cell lung carcinoma (SCLC) is a highly aggressive malignancy with few available treatment options.1, 2 Pathologically, SCLC is characterized by distinct morphology, expression of neuroendocrine (NE) markers and exceptionally high proliferation rate.3 The hallmarks of SCLC and small cell carcinomas arising in other organs include overall histologic homogeneity, with only limited variability under light microscopy. Only two subtypes of SCLC are recognized in standard pathologic classifications – one in its pure form and another combined with non-SCLC (NSCLC) or large cell neuroendocrine carcinoma (LCNEC).3 Although expression of NE markers is well known to be variable in SCLC,4 historically the extent of NE marker expression has not been regarded as biologically significant and was not known to be associated with any specific tumor characteristics. Genomically, SCLC is also a highly homogeneous disease, with the vast majority of cases characterized by inactivating RB1 and TP53 alterations.5, 6
Biological heterogeneity of SCLC has started to emerge only recently. First, recent studies, based primarily on pre-clinical models utilizing SCLC cell lines, genetically engineered mouse models (GEMMs) and patient-derived xenografts (PDXs), have identified distinct subtypes of SCLC defined by divergent gene expression programs driven by neuronal transcription factors achaete-scute homologue 1 (ASCL1) and neurogenic differentiation factor 1 (NEUROD1).7 ASCL1-high tumors were suggested to be associated with high expression of NE markers,5, 8-11 while NEUROD1-high tumors were associated with lower overall NE marker expression.8-12 However, the characteristics of NEUROD1-high SCLC are not firmly established given that closer similarity to ASCL1-high tumors was reported in several later studies.13, 14 Subsequently, a subset of ASCL1/NEUROD1 double-negative, so called “non-NE” SCLC, was found to express and show dependence on POU class 2 homeobox 3 (POU2F3) – a marker of chemosensory tuft cells, which in the lung airways are also known as brush cells.15-17 Finally, yes-associated protein 1 (YAP1), a transcriptional regulator in the HIPPO growth signaling pathway, was found to be preferentially expressed in a subset of non-NE SCLC;5, 18 however, its role as a subtype-defining transcriptional driver is not well established. Pre-clinical studies suggested distinct therapeutic vulnerabilities in the novel marker-defined subtypes of SCLC.12, 15, 19-21 Furthermore, several molecules with suggested roles in the selection of emerging therapeutic agents, including Delta-like ligand 3 (DLL3) – an inhibitor of NOTCH signaling pathway whose expression is regulated by ASCL1 and associated with NE phenotype,10, 14, 22, 23 were found to be differentially distributed among SCLC subtypes.9, 10, 12, 24 Overall, the emerging data on molecular heterogeneity of SCLC holds promise for biomarker-driven personalized therapeutic approaches for this aggressive disease.25, 26
As a result of the aforementioned studies, a recent consensus proposal suggested grouping SCLC into four subtypes defined by RNA expression of ASCL1, NEUROD1, POU2F3 and YAP1, referring to these respectively as SCLC-A, SCLC-N, SCLC-P, and SCLC-Y.7 Although there is a substantial volume of data on SCLC subtypes in pre-clinical models, there is currently very limited information on expression of a full set of these markers in the native clinical samples of SCLC, as well as pathologic characteristics associated with these subtypes. In addition, while SCLC subtypes have been defined based on transcriptional profiling, the expression of the subtype-defining markers has not been characterized in detail at the protein level. In this study, we analyzed the protein expression of ASCL1, NEUROD1, POU2F3 and YAP1 as measured by immunohistochemistry (IHC) in a large cohort of clinical samples of SCLC (N = 174). We assessed the distribution of these markers and comprehensively analyzed associated pathologic characteristics. This consisted of the analysis of morphologic features and standard diagnostic markers of SCLC, including conventional NE markers (synaptophysin, chromogranin A, CD56 and INSM1) and other classic markers of SCLC (TTF-1/NKX2-1 and Ki-67 proliferation marker). We also examined expression of DLL3 as both an additional marker of NE differentiation and a therapeutic target.
MATERIALS AND METHODS:
Sample selection
The study was performed with the approval of the institutional review board of Memorial Sloan Kettering Cancer Center, New York (MSKCC). The specimens included in the analysis comprised 122 clinical samples of SCLC diagnosed at MSKCC primarily during the period of January 2017 and January 2020, with a minority of samples diagnosed prior to these dates. In addition, 52 SCLC were analyzed in tissue microarrays (TMA). Samples were included in the analysis if an evaluable result was available for at least one of the subtype-defining markers (ASLC1, NEUROD1, POU2F3 or YAP1).
Immunohistochemistry
All cases were analyzed for novel markers of SCLC subtypes (ASCL1, NEUROD1, POU2F3 and YAP1) as well as conventional markers of SCLC (synaptophysin, chromogranin A, CD56/NCAM, INSM1, Ki-67 and TTF-1). A subset of cases (TMA only) was also analyzed for DLL3. Detailed IHC protocols and total number of cases with evaluable results for each marker are summarized in Supplementary Tables 1 and 2, respectively.
With the exception of POU2F3, all IHC protocols were performed using previously established methods. POU2F3 protocol was developed using a commercial antibody (clone 6D1, Santa Cruz), and confirmed to show specific strong nuclear labeling in scattered intramucosal cells of gastrointestinal tract and pulmonary bronchial epithelium.
IHC scoring criteria
Detailed scoring criteria for each marker are summarized in Supplementary Table 1. Expression of markers was recorded by two parameters: percent of positive cells (1-100%) and intensity of labeling (1 = weak, 2 = moderate, 3 = strong). For NE markers and DLL3, a histoscore (H-score) was derived by multiplying the percent positivity by intensity score yielding a range of possible H-scores of 0 - 300.27, 28 Ki-67 index was recorded as a percentage of positive cells, and TTF-1 was scored as either positive (any extent and intensity of labeling) or negative. To determine the “combined NE score”, the H-scores for chromogranin A, synaptophysin, CD56 and INSM1 were combined, and an average combined H-score was derived. Only cases with all 4 NE markers available were included in combined NE score. For descriptive purposes, for individual markers, H-scores of ≤50 were regarded as “low”, and those >50 as “high”. For combined NE score, scores of ≤150 were regarded as “NE-low” and those of >150 as “NE-high”. To define the dominant phenotype for cases with expression of both ASCL1 and NEUROD1, cases with higher ASCL1 H-scores than NEUROD1 were regarded as ASCL1-dominant and vice versa. In SCLC with combined SCLC and NSCLC components, IHC scores reflect expression exclusively in SCLC component.
TMA construction
SCLC TMA was constructed in the Pathology Core Laboratory, Precision Pathology Biobanking Center (PPBC), MSKCC using the automated TMA Grand Master™ (3D Histech, Hungary) and TMA Control software (Version 2.4). The H&E slides were reviewed to select most viable tumor areas, and corresponding areas in the paraffin block were punched as 1.0 mm cores (2 cores per case). A custom TMA layout was designed using the TMA Control software.
Statistical analysis
JMP version 14.0 software (SAS Institute) was used for statistical evaluation of the data. Two-tailed t-test was used for analysis of continuous variables (H-scores, Ki-67 proliferative index, etc.), while the likelihood-ratio χ2 test was used for analysis of categorical data.
RESULTS:
Patient and sample characteristics
Detailed patient and sample characteristics for 174 SCLC cases in this study are summarized in Supplementary Table 3. The patients included 96 females (55%), had an average age of 67 years, and an average smoking history of 45 pack-years. Specimens comprised 43 (25%) primary tumor resections, 105 (60%) biopsies, and 26 (15%) fine needle aspirates. Of biopsy/cytology samples, 57 (44%) were from the primary lung tumors and 74 (56%) from metastatic sites, including intra-thoracic lymph nodes (N = 40) and distant metastases (N = 34). Histologically, 82% of cases were pure SCLC, whereas 18% were combined, including combinations of SCLC with adenocarcinoma (7%), squamous cell carcinoma (3%) and LCNEC (9%).
Individual expression of ASCL1, NEUROD1, POU2F3 and YAP1
As summarized in Table 1, expression of ASCL1, NEUROD1, POU2F3 and YAP1 at any level was detected in 80%, 56%, 8% and 33% of tumors, respectively. The mean H-score in positive cases was 173 (range 4-300) for ASCL1, 95 (range 1-300) for NEUROD1, 137 (range 6-280) for POU2F3 and 22 (range 1-100) for YAP1.
Table 1.
Expression of individual subtype-defining markers in SCLC
| Marker | N tumors tested |
Total cases with expression N (%) |
H-score Range N (% cases with expression) |
H-score Mean (full range) |
|||
|---|---|---|---|---|---|---|---|
| 1 – 10 | >10 – 50 | >50 – 150 | >150 | ||||
| ASCL1 | 160 | 128 (80) | 2 (2) | 8 (6) | 41 (32) | 77 (60) | 173 (4 – 300) |
| NEUROD1 | 163 | 91 (56) | 18 (20) | 25 (28) | 24 (26) | 24 (26) | 95 (1 – 300) |
| POU2F3 | 159 | 12 (8) | 1 (8) | 0 (0) | 7 (58) | 4 (34) | 137 (6-280) |
| YAP1 | 164 | 54 (33) | 22 (41) | 28 (52) | 4 (7) | 0 (0) | 22 (1 – 100) |
Among positive cases, expression of either ASCL1 or POU2F3 was typically high, with H-score >50 seen in 92% of cases with each marker. NEUROD1 showed a broader range of expression, with similar distribution of high expressors (H-score >50 in 52% of cases) and low expressors (48% of cases). In contrast, YAP1 expression was generally low with only 4 cases (7%) above an H-scores of 50, and none above an H-score of 100.
SCLC subtypes defined by relative expression of ASCL1 and NEUROD1
Results for both ASCL1 and NEUROD1 were available for a total of 159 SCLC, yielding the following distribution: ASCL1-only (41%), NEUROD1-only (8%), ASCL1/NEUROD1 double-positive (37%), and ASCL1/NEUROD1 double-negative (14%) (Figure 1A). Dual-high expression (both ASCL1 and NEUROD1 H-score of >50) was seen in 35 (22%) tumors.
Figure 1. ASCL1 and NEUROD1 co-expression profiles.
(A) Pie chart illustrating the expression patterns of ASCL1 [A] and NEUROD1 [N] in SCLC. (B) Pie chart illustrating the distribution of marker-dominant subtypes: ASCL1-dominant (ASCL1 H-score > NEUROD1 H-score), NEUROD1-dominant (NEUROD1 H-score > ASCL1 H-score), and double-negative (negative for both markers). (C) Dot-plot depicting the H-score difference between ASCL1 and NEUROD1 (ΔH-score): each dot represents an individual case with its corresponding ΔH-score on the y-axis. All positive values represent ASCL1-dominant tumors, and all negative values represent NEUROD1-dominant ones. Bracket indicates a minority of cases with close scores for both markers (ΔH-score ≤50). (D) A case with dual-high ASCL1 and NEUROD1, illustrating that both markers are co-expressed in the same cell population.
The distribution of SCLC defined by the relative dominance of ASCL1 and NEUROD1 was as follows: ASCL1-dominant 69%, NEUROD1-dominant 17% and double-negative 14% (Figure 1B). Despite frequent co-expression of ASCL1 and NEUROD1, in most of the cases (120/137; 88%) one of the markers was strongly dominant over the other (H-score differential of >50 points) (Figure 1C). Cases with near-equivalent expression mostly occurred in the low end of expression of both markers.
Microscopic examination of tumors with dual-high ASCL1 and NEUROD1 expression revealed that all but one case had both markers co-expressed in same tumor cell populations (Figure 1D). Only a single case exhibited ASCL1 and NEUROD1 expression in spatially distinct sub-populations (Supplementary Figure 1).
ASCL1/NEUROD1-defined groups and expression of POU2F3 and YAP1
Results for ASCL1+NEUROD1+POU2F3 were available for 152 SCLC. Strikingly, POU2F3 expression was nearly exclusively restricted to ASCL1/NEUROD1-double-negative tumors (p < 0.0001, Figure 2A), of which 45% (10/22) were POU2F3-positive. Conversely, there was only a single case with POU2F3 expression in non-double-negative tumors. Notably, this was the aforementioned unique case that contained distinct geographic regions with divergent ASCL1/NEUROD1 immunoprofiles; this case also contained a third distinct area with POU2F3 expression in the absence of ASCL1 and NEUROD1 expression (Supplementary Figure 1). Thus, at an individual cell level, expression of POU2F3 was strictly mutually exclusive of both ASCL1 and NEUROD1 in all cases.
Figure 2. POU2F3 and YAP1 expression in ASCL1/NEUROD1-defined subtypes.

Dot-plots depicting POU2F3 (A) and YAP1 (B) expression in ASCL1-dominant (ASLC1), NEUROD1-dominant (NEUROD1) and double-negative (DN) tumors. The superimposed diamond plots display the group means (central horizontal lines), with the diamond height representing the 95% confidence interval (CI), and overlap marks drawn within the diamond at √2/2 x CI/2 above or below the mean, such that when the interval between these marks in one group overlaps with the mean of another group, it indicates that these groups are not different at the 95% confidence level. Thick gray lines indicate standard deviations. Gray arrow in A indicates an exceptional tumor with divergent immunoprofiles in different areas, in which POU2F3 expression was limited to a sub-population of cells lacking any NEUROD1 or ASCL1 expression (see Supplementary Figure 1). Inset in B shows YAP1 scores in the DN subset with or without POU2F3 expression. Table columns in B correspond to ASCL1, NEUROD1, DN, POU2F3 and DN, NOS. NOS: not otherwise specified; μ ± σ: mean ± standard deviation
Results for ASCL1+NEUROD1+YAP1 were available for 151 SCLC. Unlike POU2F3, YAP1 expression was distributed widely in all subtypes (Figure 2B). Although YAP1 was enriched in ASCL1/NEUROD1-negative tumors, its distribution in double-negative cases with vs without POU2F3 was similar, which together with its wide distribution in other subtypes precluded delineation of a distinct SCLC subtype defined by YAP1.
Based on the above findings, we grouped the tumors as ASCL1-dominant, NEUROD1-dominant, ASCL1/NEUROD1-double negative with POU2F3 expression (POU2F3) and ASCL1/NEUROD1-double negative, not otherwise specified (NOS). In following sections, we compared immunophenotypic and histologic characteristics of these subtypes. Separate analysis for ASCL1-only vs NEUROD1-only vs double-positive subtypes is shown in Supplementary Figure 2.
ASCL1/NEUROD1/POU2F3-defined subtypes and expression of conventional markers of SCLC
We next examined the relationship between the ASCL1/NEUROD1/POU2F3-defined subtypes and the expression of standard diagnostic markers of SCLC, including conventional markers of NE differentiation (synaptophysin, chromogranin A, CD56 and INSM1), Ki-67 proliferation marker and TTF-1. Strikingly, expression of conventional NE markers - analyzed as a combined NE score for the four markers (see Methods) - was significantly lower in ASCL1/NEUROD1-double-negative compared with ASCL1- or NEUROD1-dominant subtypes (P<0.0001) (Figure 3A). Namely, the mean combined NE score in ASCL1- and NEUROD1-dominant subtypes was 196 and 191, respectively, while in POU2F3 and NOS cases it was 76 and 89, respectively. Overall, 100% of POU2F3 and 83% of NOS cases had combined NE H-score of ≤150 (“NE-low”) compared to only 20% of ASCL1- and NEUROD1-dominant cases. When analyzed individually, all 4 NE markers were expressed at lower levels in POU2F3 and NOS subtypes (data not shown). Furthermore, the number of expressed NE markers was significantly higher in the ASCL1- and NEUROD1-dominant subtypes compared to POU2F3 and NOS subtypes (p<0.0001), with all 4 markers typically expressed in the former versus an average of ~2 in the latter subtypes (Figure 3B). Notably, none of the ASCL1/NEUROD1-double-negative tumors were entirely negative for conventional NE markers, since at least one NE marker was expressed at least weakly or focally in all POU2F3 and NOS cases. For ASCL1- vs NEUROD1-dominant subtypes, the extent and number of expressed NE markers was equivalent. Similarly, analysis of ASCL1-only vs NEUROD1-only subtypes also revealed equivalent extent and number of expressed NE markers (Supplementary Figure 2D and 2E, respectively).
Figure 3. Association of SCLC subtypes with classical markers of SCLC, DLL3 and tumor histology.

Dot-plots with superimposed diamond plots (see Figure 2 legend for details) and bar graphs illustrating (A) NE marker expression (combined NE score; see Methods), (B) the average number of positive NE markers, (C) Ki-67 proliferative index, (D) TTF-1 expression, (E) DLL3 expression, and (F) tumor histology in SCLC subtypes.
Abbreviations: ASCL1 = ASCL1-dominant; DN, NOS = ASCL1/NEUROD1-double-negative, not otherwise specified; DN, POU2F3 = ASCL1/NEUROD1-double-negative, POU2F3-positive; Comb = combined; NE = neuroendocrine; NEUROD1 = NEUROD1-dominant; Tot = total; μ ± σ: mean ± standard deviation
Analysis of Ki-67 revealed equivalent distribution in all groups, with mean proliferation indices of 88%, 90% and 89% and 86% in ASCL1-dominant, NEUROD1-dominant, POU2F3 and NOS subtypes, respectively (Figure 3C). Conversely, TTF-1 expression was strongly linked with ASCL1: most SCLC with ASCL1 expression were TTF-1-positive, whereas tumors with NEUROD1-only expression were entirely TTF-1-negative (Figure 3D and Supplementary Figure 2F). Accordingly, NEUROD1-dominant subtype had substantially lower rate of TTF-1 expression compared to ASCL1-dominant subtype (Figure 3D). Nearly all POU2F3 and most of NOS tumors were TTF-1-negative.
ASCL1/NEUROD1/POU2F3-defined subtypes and expression of DLL3
Given previously reported association of DLL3 with ASCL1-high SCLC and its role as a marker associated with NE-high phenotype, in an exploratory analysis, we examined DLL3 expression in a subgroup of SCLC typed for other markers using a TMA (N = 41 with evaluable results). Strikingly, DLL3 was entirely negative in ASCL1/NEUROD1-double-negative tumors compared to consistently high expression in both ASCL1- and NEUROD1-dominant groups (p <0.0001, Figure 3E). Expression of DLL3 was equivalent in ASCL1- vs NEUROD1-dominant subtypes. See Supplementary Figure 3 for an illustrative example.
Clinicopathologic and histologic characteristics of ASCL1/NEUROD1/POU2F3-defined subtypes
Comparison of patient and tumor characteristics, including age, gender, pack-year smoking history, tumor site (primary vs metastatic), site of metastasis (lymph node vs other organ) and specimen type revealed similar distribution in SCLC subtypes (Supplementary Table 4).
We also confirmed that histologically, all tumors in ASCL1-dominant, NEUROD1-dominant, POU2F3 and NOS subtypes represented small cell carcinoma of either pure or combined subtype.
However, we found that POU2F3 and NOS subtypes were significantly enriched in combined histology (Figure 3F). While combined histology comprised only 14% and 11% of ASCL1- and NEUROD1-dominant subtypes, respectively, POU2F3 and NOS subtypes were combined in 50% and 45% of cases, respectively. In all cases, SCLC components of combined tumors had classic histologic features of SCLC, and expression of ASCL1, NEUROD1 and POU2F3 was present exclusively or primarily in SCLC components. Histologic subtype of NSCLC components (LCNEC vs adenocarcinoma vs squamous cell carcinoma) was distributed similarly in different subtypes (Supplementary Table 4).
Lastly, given prior suggestion of genomic similarly of some ASCL1/NEUROD1-double-negative SCLC to squamous cell carcinoma,29 we confirmed that these tumors lacked evidence of squamous differentiation morphologically and consistently lacked the expression of squamous master regulator p40 (ΔNp63).
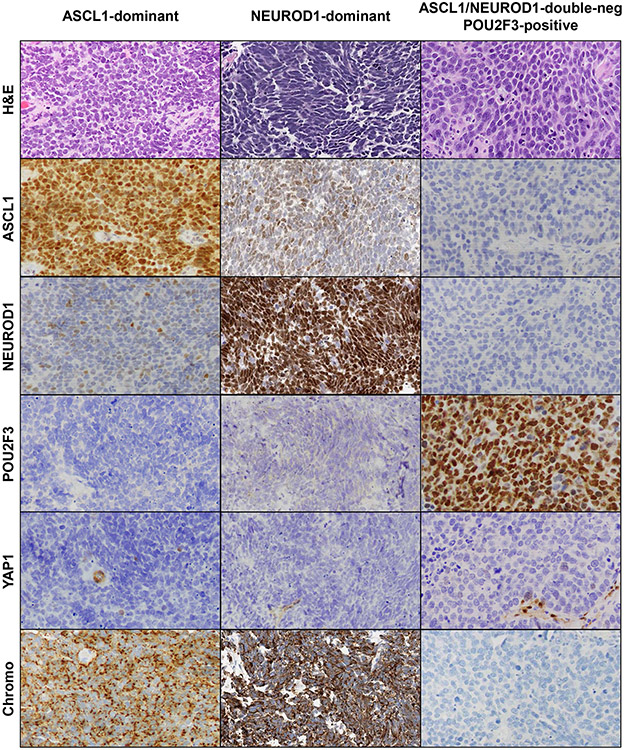
Histologic illustration of marker-defined SCLC subtypes is provided in Figure 4.
Figure 4. Histology illustration.
Examples of SCLC subtypes defined by ASCL1, NEUROD1 and POU2F3 expression. H&E illustrates classic histologic features of SCLC in all subtypes. Low-level co-expression of NEUROD1 is seen in the ASCL1-dominant case, and low-level ASCL1 co-expression is seen in the NEUROD1-dominant case. YAP1 is negative in tumor cells, but labeling is seen in benign stromal and endothelial cells. Expression of chromogranin (Chromo) illustrates high expression of neuroendocrine markers in ASCL1 and NEUROD1 subtypes, and low expression in the double-negative subtypes (for illustrated POU2F3-positive case, INSM1 and CD56 were expressed; not shown).
Correlates of YAP1 expression
Lastly, we analyzed correlates of YAP1 expression in SCLC. As shown in Figure 5A, YAP1 expression was inversely associated with NE marker expression as reflected by lower combined NE score, especially in tumors with higher YAP1 H-scores (>50). Furthermore, YAP1 expression was associated with combined SCLC histology. In particular, all tumors with higher level of YAP1 expression (H-score >50) had combined histology compared to 18% of YAP-low or negative tumors (p < 0.001). In the combined SCLC, expression of YAP1 was consistently high in the NSCLC components, and its expression in associated SCLC components ranged from absent to variably retained (Figure 5B).
Figure 5. Correlates of YAP1 expression in SCLC and example of YAP1 expression in a case of combined small cell carcinoma and adenocarcinoma.
(A) Comparison of ASCL1, NEUROD1, POU2F3, conventional markers of SCLC, and histology in tumors with differential YAP1 expression. (B) A case of combined small cell carcinoma and adenocarcinoma illustrating strong YAP1 expression in the adenocarcinoma component with weak focal staining in the adjacent SCLC component (highlighted in the high-power inset). In the background, strongly YAP1-positive cells are endothelial and stromal cells.
DISCUSSION:
In this study, using a large cohort (N = 174) of SCLC clinical samples we analyzed the protein expression of ASCL1, NEUROD1, POU2F3 and YAP1, and assessed their association with tumor histology and expression of standard markers of SCLC. This included 1) conventional markers of neuroendocrine differentiation - synaptophysin, chromogranin A, CD56 and INSM1 - which are expressed at varied levels in virtually all SCLC, 2) transcriptional regulator TTF-1 (NKX2-1), which is expressed in ~90% of SCLC, and 3) Ki-67 (MIB1) proliferation index marker. In a smaller exploratory subset, we also examined the expression of DLL3, given prior data on its distinctive distribution in ASCL1/NEUROD1-defined subtypes and its link with markers of NE differentiation. Our findings confirm some of the observations from experimental models regarding the predicted features of marker-defined subtypes, but also reveal higher level of marker expression heterogeneity and associated phenotypes in patient samples.
Overall, we confirm that the vast majority of SCLC is characterized by dominant expression of either ASCL1 (69%) and/or NEUROD1 (17%), while a minor subset of SCLC (14%) lacks either of these regulators. We found that YAP1 had distinctly low expression and did not define a distinct subtype of SCLC. Conversely, POU2F3 was uniquely associated with ASCL1/NEUROD1-double-negative subtype, comprising 7% of SCLC, while the rest of double-negative tumors (7% of SCLC) remained with no identified dominant transcriptional regulator (i.e. not otherwise specified; NOS). We found highly distinctive pathologic and immunophenotypic characteristics of SCLC defined by the presence vs absence of ASCL1NEUROD1. In particular, prior studies have suggested that expression of markers of NE differentiation, DLL3 and TTF-1 represents a coordinated “NE program” in SCLC.10, 12, 14 As such, we found that SCLC with either ASCL1 and/or NEUROD1 expression was associated with a high NE program (NE markerhigh/DLL3high/TTF-1high), whereas POU2F3 and NOS subtypes were associated with a low NE program (NE markerlow/DLL3 low/TTF-1low).
An important clarification provided by our data relates to the degree of ASCL1 and NEUROD1 co-expression in clinical samples of SCLC. In several experimental models, ASCL1 and NEUROD1 were found to have predominantly exclusive expression, with only low degree of co-expression.5, 7, 9, 10, 29-31 Conversely, we found significant level of co-expression, with 37% of SCLC expressing both ASCL1 and NEUROD1, and 22% being dual-high expressors (H-score >50 for both markers). Notably, our data are closely in line with a study by Zhang et al, in which using a smaller set of human SCLC FFPE samples (N = 81), the authors also found frequent (19.8%) ASCL1 and NEUROD1 dual-high expression.14 It is possible that in experimental models there is clonal selection for a dominant transcription factor and inhibition of the minor factor under culture or PDX conditions, as supported by experimental evidence of SCLC subtype plasticity.31, 32
Notably, in the series by Zhang et al,14 the question of whether ASCL1 and NEUROD1 were co-expressed in the same cell populations or exhibited subclonal expression in distinct tumor cell populations was not addressed. In a study of circulating-tumor-cell-derived xenografts (CDX), it was suggested that dual expression was present in distinct cell subpopulations.13 Conversely, our study is the first to document that in native patient samples, nearly all cases of dual-high expression represent co-expression within the same cell populations, as illustrated in Figure 1D, while only a single case exhibited sub-clonal areas with divergent ASCL1 and NEUROD1 (as well as POU2F3) expression.
Several distinctive characteristics of NEUROD1-high SCLC have been suggested in prior studies compared to ASCL1-high tumors, including highly divergent global gene expression, with lower expression of NE genes and DLL3.12, 24 Conversely, we found that in patient samples, NEUROD1-dominant SCLC expressed high levels of NE markers and DLL3, equivalent to those of ASCL1-dominant tumors. Analysis of NEUROD1-associated characteristics was complicated by the fact that most (83%) of NEUROD1-dominant tumors co-expressed ASCL1, and only a small subset of cases had exclusive NEUROD1 expression. However, our findings in these NEUROD1-only tumors confirmed that they also had high expression of NE markers and DLL3. This discrepancy could reflect RNA versus protein level analysis in the current versus prior studies, respectively, in part due to the wider dynamic range of gene expression measurements when compared to IHC. Nonetheless, we note that similar to the results of this study, other recent RNA-based investigations also suggested that NEUROD1-high SCLC have greater transcriptional overlap with ASCL1-high tumors than previously proposed.13, 14 Interestingly, we did find that TTF-1 was consistently negative in NEUROD1-only tumors but positive in nearly all ASCL1-dominant tumors and NEUROD1-dominant tumors with variable levels of ASCL1 co-expression, which is in line with the known co-dependence of TTF-1 and ASCL1 expression.33 This might suggest that distinct transcriptional program may correspond to SCLC with exclusive NEUROD1 expression as measured by IHC. However, further studies will be required to clarify IHC-based criteria for SCLC-N vs SCLC-A subtypes associated with globally distinct transcriptional programs as defined in mRNA-based studies using parallel mRNA and protein analysis.9, 10, 30
One of the major findings in this study is the confirmation of a highly distinct nature of the POU2F3-positive SCLC, defined by POU2F3 expression and strict lack of ASCL1 and NEUROD1. POU2F3 is a transcription factor required for the generation and chemosensory and immune functions of specialized tuft cells in the skin, oropharyngeal, gastrointestinal, and respiratory tracts.34-42 It has been proposed that POU2F3 defines a distinct subset of SCLC arising from or recapitulating the differentiation of the tuft cells.15, 16 However, the singular case with distinct ASCL1-positive, NEUROD1-positive and POU2F3-positive components calls into question the suggestion that SCLC-P might derive from a different cell of origin from other SCLC subtypes. We confirm that POU2F3-positive SCLCs are characterized by NE markerlow/TTF-1low/DLL3low profile. Importantly, we also confirm that pathologically, POU2F3-positive tumors represent true SCLC as defined by morphology and extremely high proliferation rate, despite the low NE markers and near-universal lack of TTF-1 expression. Enrichment of POU2F3 expression in combined SCLC suggests either greater morphologic plasticity or closer ontogenetic relationship with NSCLC than ASCL1/NEUROD1 subtypes. Similar to conventional SCLC, we confirmed that POU2F3-expressing tumors lack RB expression (data not shown).
YAP1 has been proposed to represent one of the subtype-defining markers of SCLC within the “non-NE” ASCL1/NEUROD1 double-negative group of tumors, associated with decreased INSM1 expression and enrichment for intact RB.7, 18 However, the role of YAP1 as a major transcriptional driver in SCLC remains unclear given that it was also reported that YAP1 expression in SCLC is consistently absent or minimal,21 and no distinct YAP1 subtype was identified in a recent study of SCLC circulating tumor cell-derived xenografts.13 Consistent with these latter observations, we also found that YAP1 expression in SCLC was either entirely absent or present only at a low level. Few cases that exhibited moderate expression of YAP1 were confined to SCLC with combined histology, where YAP1 was strongly expressed in the NSCLC component and focally retained YAP1 expression in the SCLC component could reflect transitional phenotype between NSCLC and SCLC in such regions. Furthermore, YAP1 did not define a distinct subgroup of SCLC, although its expression was mildly elevated in the ASCL1/NEUROD1 double-negative tumors and was associated with low expression of NE markers. Overall, the role of YAP1 as a subtype-defining marker in SCLC will require clarification in future studies.
In our series, 7% of SCLC lacked a known transcriptional regulator as they were negative for ASCL1, NEUROD1 and POU2F3, and were generally either negative or low for YAP1. Overall, these tumors were similar to the POU2F3 subtype in that they were NE markerlow/TTF-1low/DLL3low and enriched in combined histology. Identification of a dominant lineage marker in this subtype of NE-low SCLC requires further study.
Another important clarification provided by our data concerns the so-called “non-NE” subtype of SCLC.7 It is known from prior IHC-based FFPE studies that some SCLC have only minimal NE marker expression.4 However, with the use of the most sensitive markers – CD56 and the recent marker INSM1 – the prevalence of entirely NE-negative SCLC is exceptionally low.4, 43, 44 These concepts are closely recapitulated by our data, which clarify that rather than being entirely “non-NE”, ASCL1/NEUROD1-double-negative tumors represent primarily SCLC with NE-low (or NE-intermediate) phenotype, indicating that they have lower extent and fewer number of expressed NE markers compared to ASCL1 and/or NEUROD1-expressing SCLC.
Dr. Gazdar and colleagues originally proposed, based primarily on SCLC cell lines, the existence of “variant” SCLC, characterized by semi-adherent larger cells compared to floating spherical aggregates of “classic” SCLC.8 However the precise translation of these concepts to FFPE tissue and clinical patient samples has not been established. In relation to marker expression, in some studies, “variant” SCLC corresponded to the ASCL1/NEUROD1 double-negative tumors, but in others to the NEUROD1-high tumors. We demonstrate enrichment for combined histology in the double-negative SCLC both with and without POU2F3 expression, raising the consideration that what has been referred to as “variant” morphology in experimental settings may reflect at least in some cases combined SCLC histology. Although we did not identify enrichment for combined or other distinctive histology in NEUROD1-expressing SCLC, it cannot be excluded that variant characteristics of some SCLC are only apparent or amplified under culture or other experimental conditions.
In summary, in the first comprehensive IHC-based study in patient samples, we confirm highly distinct characteristics of SCLC with ASCL1 and/or NEUROD1 expression (86%) versus SCLC lacking these markers (14%). The former are associated with a high “NE program” (NE markerhigh/TTF-1high/DLL3high) and pure SCLC histology, whereas the latter demonstrate a low “NE program” and enrichment in combined histology. We also confirm a highly distinctive nature of POU2F3-expressing tumors, which account for 7% of SCLC. Further studies are warranted to determine whether expression-based subtypes of SCLC are associated with distinct patient outcomes and/or predict distinct therapeutic vulnerabilities.
Supplementary Material
ACKNOWLEDGEMENTS:
This work was supported in part by the Fiona and Stanley Druckenmiller Center for Lung Cancer Research, R01 CA19793604, R01 CA213448-01 and U01 CA213359-01. We acknowledge the infrastructural support from the National Cancer Institute Cancer Center Core Grant P30-CA008748.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare that there are no conflicts of interests.
REFERENCES:
- 1.Hann CL, Rudin CM. Management of small-cell lung cancer: incremental changes but hope for the future. Oncology (Williston Park) 2008;22:1486–1492. [PMC free article] [PubMed] [Google Scholar]
- 2.Sabari JK, Lok BH, Laird JH, et al. Unravelling the biology of SCLC: implications for therapy. Nat Rev Clin Oncol 2017;14:549–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Travis WD, Brambilla E, Burke A, et al. WHO classification of tumours of the lung, pleura, thymus and heart. Lyon: International Agency for Research on Cancer; 2015. [DOI] [PubMed] [Google Scholar]
- 4.Rekhtman N Neuroendocrine tumors of the lung: an update. Arch Pathol Lab Med 2010;134:1628–1638. [DOI] [PubMed] [Google Scholar]
- 5.George J, Lim JS, Jang SJ, et al. Comprehensive genomic profiles of small cell lung cancer. Nature 2015;524:47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peifer M, Fernández-Cuesta L, Sos ML, et al. Integrative genome analyses identify key somatic driver mutations of small-cell lung cancer. Nature Genetics 2012;44:1104–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rudin CM, Poirier JT, Byers LA, et al. Molecular subtypes of small cell lung cancer: a synthesis of human and mouse model data. Nature Reviews Cancer 2019;19:289–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gazdar AF, Carney DN, Nau MM, et al. Characterization of variant subclasses of cell lines derived from small cell lung cancer having distinctive biochemical, morphological, and growth properties. Cancer Res 1985;45:2924–2930. [PubMed] [Google Scholar]
- 9.Mollaoglu G, Guthrie MR, Böhm S, et al. MYC Drives Progression of Small Cell Lung Cancer to a Variant Neuroendocrine Subtype with Vulnerability to Aurora Kinase Inhibition. Cancer Cell 2017;31:270–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Borromeo MD, Savage TK, Kollipara RK, et al. ASCL1 and NEUROD1 Reveal Heterogeneity in Pulmonary Neuroendocrine Tumors and Regulate Distinct Genetic Programs. Cell Rep 2016; 16:1259–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Calbo J, van Montfort E, Proost N, et al. A functional role for tumor cell heterogeneity in a mouse model of small cell lung cancer. Cancer Cell 2011;19:244–256. [DOI] [PubMed] [Google Scholar]
- 12.Cardnell RJ, Li L, Sen T, et al. Protein expression of TTF1 and cMYC define distinct molecular subgroups of small cell lung cancer with unique vulnerabilities to aurora kinase inhibition, DLL3 targeting, and other targeted therapies. Oncotarget 2017;8:73419–73432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Simpson KL, Stoney R, Frese KK, et al. A biobank of small cell lung cancer CDX models elucidates inter- and intratumoral phenotypic heterogeneity. Nature Cancer 2020;1:437–451. [DOI] [PubMed] [Google Scholar]
- 14.Zhang W, Girard L, Zhang Y-A, et al. Small cell lung cancer tumors and preclinical models display heterogeneity of neuroendocrine phenotypes. Translational Lung Cancer Research 2018;7:32–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang YH, Klingbeil O, He XY, et al. POU2F3 is a master regulator of a tuft cell-like variant of small cell lung cancer. Genes Dev 2018;32:915–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reid L, Meyrick B, Antony VB, et al. The mysterious pulmonary brush cell: a cell in search of a function. Am J Respir Crit Care Med 2005;172:136–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nevo S, Kadouri N, Abramson J. Tuft cells: From the mucosa to the thymus. Immunol Lett 2019;210:1–9. [DOI] [PubMed] [Google Scholar]
- 18.McColl K, Wildey G, Sakre N, et al. Reciprocal expression of INSM1 and YAP1 defines subgroups in small cell lung cancer. Oncotarget 2017;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Augert A, Eastwood E, Ibrahim AH, et al. Targeting NOTCH activation in small cell lung cancer through LSD1 inhibition. Sci Signal 2019;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rudin CM, Poirier JT, Senzer NN, et al. Phase I clinical study of Seneca Valley Virus (SVV-001), a replication-competent picornavirus, in advanced solid tumors with neuroendocrine features. Clin Cancer Res 2011;17:888–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ito T, Matsubara D, Tanaka I, et al. Loss of YAP1 defines neuroendocrine differentiation of lung tumors. Cancer Sci 2016;107:1527–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.George J, Walter V, Peifer M, et al. Integrative genomic profiling of large-cell neuroendocrine carcinomas reveals distinct subtypes of high-grade neuroendocrine lung tumors. Nature Communications 2018;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hermans BCM, Derks JL, Thunnissen E, et al. DLL3 expression in large cell neuroendocrine carcinoma (LCNEC) and association with molecular subtypes and neuroendocrine profile. Lung Cancer 2019;138:102–108. [DOI] [PubMed] [Google Scholar]
- 24.Saunders LR, Bankovich AJ, Anderson WC, et al. A DLL3-targeted antibody-drug conjugate eradicates high-grade pulmonary neuroendocrine tumor-initiating cells in vivo. Science Translational Medicine 2015;7:302ra136–302ra301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Poirier JT, George J, Owonikoko TK, et al. New Approaches to SCLC Therapy: From the Laboratory to the Clinic. J Thorac Oncol 2020;15:520–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taniguchi H, Sen T, Rudin CM. Targeted Therapies and Biomarkers in Small Cell Lung Cancer. Front Oncol 2020;10:741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fedchenko N, Reifenrath J. Different approaches for interpretation and reporting of immunohistochemistry analysis results in the bone tissue – a review. Diagnostic Pathology 2014;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McCarty KS Jr., Miller LS, Cox EB, et al. Estrogen receptor analyses. Correlation of biochemical and immunohistochemical methods using monoclonal antireceptor antibodies. Arch Pathol Lab Med 1985;109:716–721. [PubMed] [Google Scholar]
- 29.Poirier JT, Gardner EE, Connis N, et al. DNA methylation in small cell lung cancer defines distinct disease subtypes and correlates with high expression of EZH2. Oncogene 2015;34:5869–5878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Poirier JT, Dobromilskaya I, Moriarty WF, et al. Selective tropism of Seneca Valley virus for variant subtype small cell lung cancer. J Natl Cancer Inst 2013; 105:1059–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wooten DJ, Groves SM, Tyson DR, et al. Systems-level network modeling of Small Cell Lung Cancer subtypes identifies master regulators and destabilizers. PLoS Comput Biol 2019;15:e1007343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ireland AS, Micinski AM, Kastner DW, et al. MYC Drives Temporal Evolution of Small Cell Lung Cancer Subtypes by Reprogramming Neuroendocrine Fate. Cancer Cell 2020;38:60–78.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Horie M, Miyashita N, Mattsson JSM, et al. An integrative transcriptome analysis reveals a functional role for thyroid transcription factor-1 in small cell lung cancer. The Journal of Pathology 2018;246:154–165. [DOI] [PubMed] [Google Scholar]
- 34.Gerbe F, Sidot E, Smyth DJ, et al. Intestinal epithelial tuft cells initiate type 2 mucosal immunity to helminth parasites. Nature 2016;529:226–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Howitt MR, Lavoie S, Michaud M, et al. Tuft cells, taste-chemosensory cells, orchestrate parasite type 2 immunity in the gut. Science 2016;351:1329–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matsumoto I, Ohmoto M, Narukawa M, et al. Skn-1a (Pou2f3) specifies taste receptor cell lineage. Nat Neurosci 2011; 14:685–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ohmoto M, Yamaguchi T, Yamashita J, et al. Pou2f3/Skn-1a is necessary for the generation or differentiation of solitary chemosensory cells in the anterior nasal cavity. Biosci Biotechnol Biochem 2013;77:2154–2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O'Leary CE, Schneider C, Locksley RM. Tuft Cells-Systemically Dispersed Sensory Epithelia Integrating Immune and Neural Circuitry. Annu Rev Immunol 2019;37:47–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schneider C, O'Leary CE, Locksley RM. Regulation of immune responses by tuft cells. Nat Rev Immunol 2019;19:584–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.von Moltke J, Ji M, Liang HE, et al. Tuft-cell-derived IL-25 regulates an intestinal ILC2-epithelial response circuit. Nature 2016;529:221–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yamaguchi T, Yamashita J, Ohmoto M, et al. Skn-1a/Pou2f3 is required for the generation of Trpm5-expressing microvillous cells in the mouse main olfactory epithelium. BMC Neurosci 2014;15:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yamashita J, Ohmoto M, Yamaguchi T, et al. Skn-1a/Pou2f3 functions as a master regulator to generate Trpm5-expressing chemosensory cells in mice. PLoS One 2017;12:e0189340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mukhopadhyay S, Dermawan JK, Lanigan CP, et al. Insulinoma-associated protein 1 (INSM1) is a sensitive and highly specific marker of neuroendocrine differentiation in primary lung neoplasms: an immunohistochemical study of 345 cases, including 292 whole-tissue sections. Modern Pathology 2019;32:100–109. [DOI] [PubMed] [Google Scholar]
- 44.Rooper LM, Sharma R, Li QK, et al. INSM1 Demonstrates Superior Performance to the Individual and Combined Use of Synaptophysin, Chromogranin and CD56 for Diagnosing Neuroendocrine Tumors of the Thoracic Cavity. The American Journal of Surgical Pathology 2017;41:1561–1569. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.