Abstract
Background
Biological agents have been used with extreme caution in children because of their possible adverse effects.
Objectives
This study used high-quality randomized controlled trials (RCTs) to provide high-level evidence to assess the effectiveness and safety of biological agents for treating children with psoriasis.
Methods
We searched PubMed, Embase, Cochrane, and Web of Science databases through October 31, 2021. We included trials reporting at least one adverse event after treatment with biological agents of patients less than 18-year-old diagnosed with psoriasis. RevMan 5.3 and Stata 15.0 software were used for meta and Bayesian analyses.
Results
Six trials with 864 participants were included in the analysis. The results showed a 2.37-fold higher response rate in all biologics groups than in the control group for psoriasis area and severity index 75 (PASI75) (RR= 2.37, P-value < 0.01, 95% confidence interval [CI] [1.22, 4.62]). Compared with placebo, the PASI75 response rates of etanercept (RR= 2.82, 95% [CI] [1.10, 7.21]), ustekinumab low dose (RR= 7.45, 95%[CI] [1.25, 44.58]), and ustekinumab high dose (RR= 7.25, 95%[CI] [1.21, 43.41]) were superior. Additionally, the incidence of total adverse reactions was 1.05 times higher for biologics than for controls, indicating a good safety profile (RR= 1.05, P-value = 0.53, 95%[CI] [0.92, 1.19]). Overall, these six high-quality randomized controlled trials suggest that biologics are effective and safe for pediatric patients with psoriasis.
Limitations
Inclusion of few relevant, high-quality RCTs.
Conclusion
The results of this study indicate that biologics can be used to treat children with moderate-to-severe psoriasis without the risk of adverse effects. Ustekinumab showed the best efficacy and the fewest adverse effects.
Keywords: biological agents, pediatric, psoriasis, adverse events, Bayesian analysis, systematic review
Introduction
Psoriasis is a common immune-mediated condition primarily characterized by skin lesions, affecting approximately 3% of the population worldwide (1), and nearly one-third of the patients present with disease symptoms before adulthood (2). Pediatric psoriasis is often highly visible and uncomfortable, and approximately 20% of children present with moderate to severe disease, which may require effective systemic therapy (3). Biologics are an important therapeutic option for moderate-to-severe psoriasis when other treatments are contraindicated or ineffective (4). Evidence confirms that the correlation between biologics and serious infections is small in the general population; however, biologics have been used in special populations, such as children, pregnant women, and the elderly, with extreme caution owing to the risk of adverse effects (5).
In 2002, etanercept was approved by the US Food and Drug Administration (FDA) to treat psoriasis. Twelve years later, in 2014, it was expanded to the treatment of pediatric patients (4–17 years old) with chronic moderate-to-severe plaque psoriasis (6). In 2021, secukinumab was approved by the FDA for the treatment of patients aged 6–18 years with moderate-to-severe plaque psoriasis. Currently, ixekizumab and ustekinumab are also approved to treat patients over six years of age with mild-to-moderate plaque psoriasis. However, relevant evaluations for utilizing biologics in childhood and adolescent psoriasis are lacking.
Biological agents are engineered monoclonal antibodies and fusion proteins capable of therapeutic action by blocking specific cytokines or cytokine receptors critical to psoriatic inflammation (7). It is speculated that TH1 and TH17 mediate psoriasis, and inhibition of TH17 is a known therapeutic strategy. Drugs targeting tumor necrosis factor(TNF)-α, interleukin(IL)-23, and IL-17 are effective for the clinical treatment of psoriasis (8). TNF-α inhibitors dominate the therapeutic market for psoriasis treatment. Furthermore, IL-12/IL-23 and IL-17 inhibitors could reduce the expansion and production of TH17, thereby suppressing the production of pro-inflammatory factors with relatively few adverse reactions (9). Accordingly, biologics are being progressively used to rapidly and effectively decrease psoriasis severity, although side effects have been noted, along with relapses after drug withdrawal.
Several studies have demonstrated the relative safety of biologics in special populations, particularly pediatric and pregnant patients (10–12). However, individual reports have limited power to characterize the risk factors for AEs in children and adolescents, and clinicians and patients are eager to receive corresponding guidance. Therefore, this review collates information from currently available studies documenting the apparent side effects of biologics, which can direct future clinical trials.
Methods
This review was conducted using the Cochrane Handbook on Systematic Review of Interventions and presented following the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) guidelines (13) ( Data Sheet 1 ).
Search strategy
Four databases (PubMed, Embase, Cochrane Library, and Web of Science) were searched from inception to October 31, 2021. We combined subject headings and free text words to retrieve all relevant studies. The following keywords were used: (“clinic” OR “clinical”) and (“Secukinumab” OR “Brodalumab” OR “Ixekizumab” OR “Ustekinumab” OR “Guselkumab” OR “Tildrakizumab” OR “Risankizumab” OR “Brazikumab” OR “Mirikizumab” OR “Etanercept” OR “Adalimumab” OR “Infliximab”) and (“children” OR “adolescent” OR “teenager” OR “young” OR “boy” OR “girl” OR “pediatric”) and (psoriasis).
Data inclusion
We determined the inclusion and exclusion criteria for the present analysis before conducting a literature search. The inclusion criteria were as follows: RCTs reporting at least one type of AE after intervention with biologics, subjects diagnosed with psoriasis and aged<18 years, regardless of sex and ethnicity.
Data extraction
Two investigators (X.C. Cai and M. Zhang) independently screened the studies according to the inclusion criteria using self-designed data extraction templates for each included study. Two authors (X.C. Cai and X.Y. Sun) assessed the risk of bias, and three authors (Y.Q. Zhou, Y. Luo, and J.L. Chen) performed data analysis and interpretation.
Outcome measures
The primary efficacy measure was the PASI 75, a quantitative rating score that measures the severity of psoriatic lesions based on area coverage and plaque appearance, including scaling, infiltration, and erythema. In addition, the PASI 50, PASI 90, PASI 100, sPGA, sPGA0/1, and DLQI 0/1 indices were used to evaluate the efficacy outcomes. The main outcome indicators for safety evaluations were AEs and serious AEs. The secondary indicators of adverse reactions were the five types of adverse reactions produced by biologics (14). This classification could help better deal with the clinical features of these side effects, identify individual and general risk factors, and direct research in this novel area of medicine.
Statistical analysis
RevMan5.3 software provided by the Cochrane Collaboration, was used to synthesize the results of the meta-analysis. Risk ratios (RR) with 95% confidence intervals (CIs) were calculated for dichotomous data. The mean difference (MD) and standard mean difference (SMD) were used for continuous data. Across trials, a fixed-effects model was used for homogeneity (I2 < 25%); otherwise, a random-effects model was used. Statistical significance was set at P-value < 0.05.
Network analysis was conducted in Stata 15.0 and using Markov Chain Monte Carlo (MCMC) simulation random-effects modeling in a Bayesian frame. For each analysis, we used three chains and generated 100,000 iterations. The first 20,000 iterations were the burn-in periods to eliminate the effect of initial values, and the last 80,000 iterations were used for sampling. The convergence was evaluated using a trace plot. Inconsistency between direct and indirect comparisons was assessed by the “node-splitting” method. The surface under the cumulative ranking (SUCRA) (%) was calculated. If SUCRA approaches 100%, the intervention is the best among the included trials; conversely, if it is close to 0%, it represents the worst intervention.
Results
Study selection and characteristics
We identified 172 studies after a preliminary search of four databases and 87 related articles (related articles and citations). After removing duplicate studies, 195 remained. Subsequently, the titles and abstracts of each study were screened, and 43 studies were available for a detailed evaluation. Of the remaining articles, 37 were excluded, of which 14 were not available in full text, 19 were non-RCTs, 2 did not report AEs, and 2 were excluded due to other reasons such as the articles being letters. Six full-text articles were included in the final analysis (15–20). All the trials were published in English ( Figure 1 ).
Figure 1.
Flowchart of study inclusion according to the PRISMA 2009 guidelines. RCTs: randomized control trials; AEs, adverse events.
Finally, six trials involving 864 participants were included in the analysis: four clinical studies compared biologics with placebo, one study compared two biologics, and one compared a biologic with methotrexate (MTX) ( Table 1 ).
Table 1.
Summary of included trials characteristics.
| Author year | Phase | Multicenter (Y/N) | Region | Enrollment number | Age limit | Severity | Sample size (M/F) | Male n% | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I | C | ||||||||||||
| Bodemer 2021 ( 15) | III | Y | Global | NCT02471144 | 6 to <18 | severe | 30/50 | 16/25 | 39.0 | ||||
| Paller 2020 ( 18) | III | Y | Europe and America | NCT03073200 | 6 to < 18 | moderate-to-severe | 52/63 | 20/36 | 42.2 | ||||
| Papp 2017 ( 17) | III | Y | South America, North America, and Europe | NCT01251614 | ≥4 to <18 | severe | 38/39 | 11/26 | 43.0 | ||||
| Landells 2015 ( 16) | III | Y | North America | NCT01090427 | 12 to 17 | moderate-to-severe | 20/17 | 34/39 | 49.1 | ||||
| Siegfried 2010 ( 20) | III | Y | American | NCT00078819 | 4 to 17 | moderate-to-severe | 32/37 | 33/36 | 49.3 | ||||
| Paller 2008 ( 19) | III | Y | American | NCT00078819 | 4 to 17 | moderate-to-severe | 55/51 | 53/52 | 51.2 | ||||
Table 1 Continued.
| Author year | Sample age (yr) | Duration of treatment (wk) | Disease duration, yr | Target | Biologics | Control | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I Mean (SD) | C Mean (SD) | I Mean (SD) | C Mean (SD) | ||||||||||
| Bodemer 2021 ( 15) | 13.5 (3.07) | 13.5 (2.94) | 52 | 5.15 (4.48) | 4.55 (3.73) | IL-17A | Secukinumab | Etanercept | |||||
| Paller 2020 ( 18) | 13.7 (4.14) | 13.1 (2.79) | 12 | 4.7 (3.26) | 4.7 (3.01) | IL-17A | Ixekizumab | placebo | |||||
| Papp 2017 ( 17) | 12.8 (4.0) | 13.4 (3.5) | 16 | 4.9 (3.6) | 5.1 (3.8) | TNF-α | Adalimumab | MTX | |||||
| Landells 2015 ( 16) | 14.9 (1.7) | 15.6 (1.5) | 12 | 5.7 (3.9) | 6.2 (5.0) | IL-23 | Ustekinumab | placebo | |||||
| Siegfried 2010 ( 20) | 13.0 | 13.0 | 12 | 5.3 | 5.9 | TNF-α | Etanercept | Placebo | |||||
| Paller 2008 ( 19) | 14.0 | 13.0 | 12 | 6.8 | 5.8 | TNF-α | Etanercept | placebo | |||||
Table 1 Continued.
| Author Year | Dose | Frequent | PASI 50 |
PASI 75 |
PASI 90 |
PASI100 | SPGA 0/1 |
SPGA 0 |
CDQLI 0/1 |
AE | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I | C | I | C | ||||||||||
| Bodemer 2021 ( 15) | 75/150/300 mg | 75/150/300 mg | 0,1,2,3,4,then Q4W | 0,1,2,3,4,then Q4W | ● | ● | ● | ● | ● | ||||
| Paller 2020 ( 18) | 0.8 mg/kg | / | Q4W | Q4W | ● | ● | ● | ● | ● | ● | ● | ● | |
| Papp 2017 ( 17) | 0.8 or 0.4 mg/kg | 0.1–0.4 mg/kg | Q2W | Q1W | ● | ● | ● | ● | ● | ||||
| Landells 2015 ( 16) | 0.75 or 0.375 mg/kg | / | weeks 0 and 4 | weeks 0 and 4 | ● | ● | ● | ● | ● | ● | |||
| Siegfried 2010 ( 20) | 0.8 mg/kg | / | Q1W | Q1W | ● | ● | ● | ||||||
| Paller 2008 ( 19) | 0.8 mg/kg | / | Q1W | Q1W | ● | ● | ● | ● | ● | ||||
Wk, week; y, year; M, male; F, female; Y, yes; N, no; I, intervention; C, control; SD, standard difference; AE, adverse event; PASI, Psoriasis Area and Severity Index; PASI 50/75/90/100, PASI score decreased by more than 50%, 75%, 90%, 100% from baseline; sPGA0/1, static physician’s global assessment achieve 0 or 1; sPGA0, static physician’s global assessment achieve 0; DLQI 0/1, Children’s Dermatology Life Quality Index achieved 0 or 1; MTX, methotrexate; Q1W, every 1 week; Q2W, every 2 weeks; Q4W, every 4 weeks; IL17A, interleukin-17A; Il-23, interleukin-23; TNF-α, tumor necrosis factor-α.
Risk of bias assessment
All the included RCTs described a specific stochastic approach. Three studies (15, 18, 20) did not report details regarding selection bias. All studies were double-blinded (participants and personnel) and were assessed in a blinded manner. We evaluated the completed data without selective reporting or other bias. In summary, the quality of the included studies was high, and the results of this meta-analysis were credible ( Figure 2 ).
Figure 2.
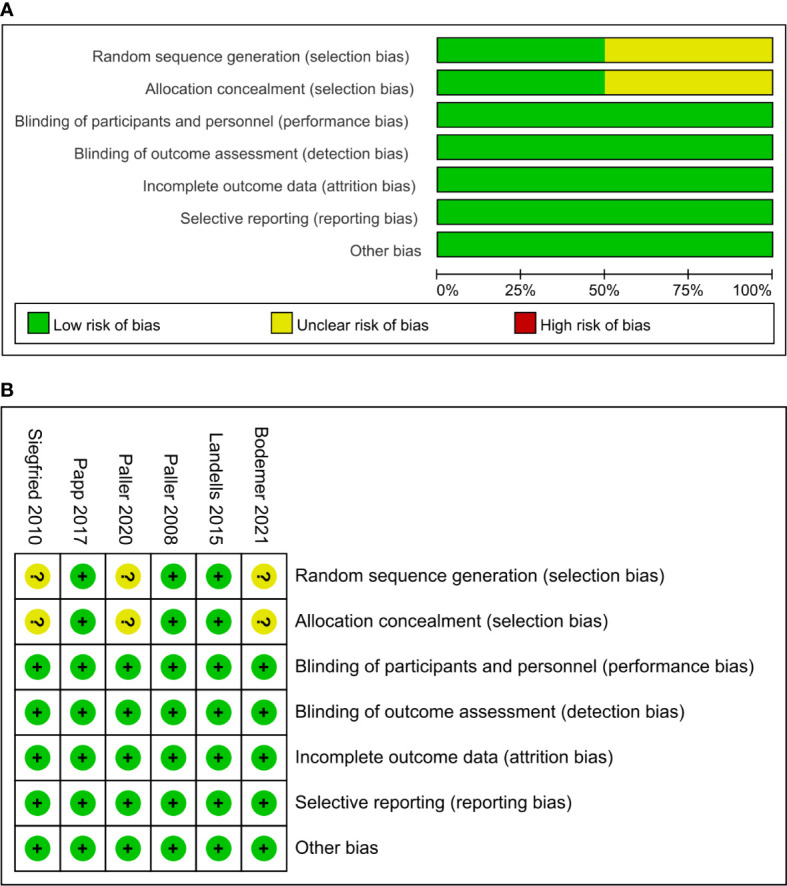
Risk of bias summary of clinical studies (A). Risk of bias graph of clinical studies (B).
Outcomes
Efficacy
Psoriasis Area and Severity Index (PASI) 75
PASI 75 indicates that the lesion score improved by 75% compared with the baseline score. In the present study, the PASI 75 was used to evaluate the primary efficacy of biologics for broader applications. We observed that the efficacy of biologics (ustekinumab, ixekizumab, and etanercept) was significantly higher than that of placebo ( Table 2 , Data Sheet 2 ). In addition, adalimumab was more effective than MTX ( Table 2 ; Data Sheet 2 ). Collectively, the biologic-treated groups presented 2.37-fold better results than the control group ( Table 2 , Data Sheet 2 ; P-value < 0.01, RR = 2.37, 95%[CI] [1.22, 4.62]).
Table 2.
Efficacy of biological agents in pediatric patients with psoriasis.
| Subgroup | Experimental | Control | Risk ratio M-H (95% CI) | Study weight, % | I2, % | P-value | ||
|---|---|---|---|---|---|---|---|---|
| Events | Total | Events | Total | |||||
| PASI 75 | ||||||||
| Ustekinumab vs. Placebo | 58 | 73 | 4 | 37 | 7.35 [2.89,18.68] | 13.6 | 0.01# | |
| Ixekizumab vs. Placebo | 102 | 115 | 14 | 56 | 3.55 [2.24,5.61] | 17.0 | 0.01# | |
| Etanercept vs. Placebo | 112 | 174 | 67 | 174 | 2.15 [0.26,18.04] | 34.6 | 98 | 0.01# |
| Secukinumab vs. Etanercept | 72 | 80 | 28 | 41 | 1.32 [1.06,1.64] | 18.1 | 0.01# | |
| Adalimumab vs. MTX | 39 | 77 | 12 | 37 | 1.56 [0.93,2.61] | 16.7 | 0.09 | |
| Total(95% CI) | 383 | 519 | 125 | 345 | 2.37 [1.22,4.62] | 100.0 | 95 | 0.01# |
| PASI 50 | ||||||||
| Etanercept vs. Placebo | 79 | 106 | 24 | 105 | 3.26 [2.26,4.71] | 46.1 | 0.01# | |
| Ixekizumab vs. Placebo | 106 | 115 | 21 | 56 | 2.46 [1.75,3.46] | 53.9 | 0.01# | |
| Total(95% CI) | 185 | 221 | 45 | 161 | 2.83 [2.20,3.63] | 100.0 | 18 | 0.27 |
| PASI 90 | ||||||||
| Ustekinumab vs. Placebo | 42 | 73 | 2 | 37 | 10.64 [2.73,41.56] | 16.5 | 0.01# | |
| Ixekizumab vs. Placebo | 90 | 115 | 3 | 56 | 14.61 [4.84,44.11] | 18.4 | 0.01# | |
| Etanercept vs. Placebo | 29 | 106 | 7 | 105 | 4.10 [1.88,8.95] | 20.7 | 0.01# | |
| Secukinumab vs. Etanercept | 64 | 80 | 21 | 41 | 1.56 [1.14,2.15] | 23.1 | 0.01# | |
| Adalimumab vs. MTX | 23 | 77 | 8 | 37 | 1.38 [0.68,2.79] | 21.2 | 0.37 | |
| Total (95% CI) | 248 | 451 | 41 | 276 | 3.85 [1.40,10.58] | 100.0 | 89 | 0.01# |
| PASI 100 | ||||||||
| Ixekizumab vs. Placebo | 57 | 115 | 1 | 56 | 27.76 [3.94,195.31] | 29.9 | 0.01# | |
| Secukinumab vs. Etanercept | 36 | 80 | 9 | 41 | 2.05 [1.10,3.83] | 40.8 | 0.02* | |
| Adalimumab vs. MTX | 11 | 77 | 1 | 37 | 5.29 [0.71,39.42] | 29.3 | 0.10 | |
| Total (95% CI) | 104 | 272 | 11 | 134 | 5.89 [0.84,41.51] | 100.0 | 80 | 0.01# |
| sPGA 0/1 | ||||||||
| Ustekinumab vs. Placebo | 50 | 73 | 2 | 37 | 12.67 [3.26,49.22] | 15.2 | 0.01# | |
| Ixekizumab vs. Placebo | 93 | 115 | 6 | 56 | 7.55 [3.53,16.16] | 19.7 | 0.01# | |
| Etanercept vs. Placebo | 56 | 106 | 14 | 105 | 3.96 [2.36,6.66] | 21.3 | 95 | 0.01# |
| Adalimumab vs. MTX | 39 | 77 | 15 | 37 | 1.25 [0.80,1.96] | 21.6 | 0.33 | |
| Total (95% CI) | 276 | 439 | 27 | 304 | 3.15 [1.26,7.86] | 100.0 | 93 | 0.01# |
| sPGA 0 | ||||||||
| Ustekinumab vs. Placebo | 29 | 73 | 1 | 37 | 14.70 [2.08,103.71] | 49.7 | 0.01# | |
| Ixekizumab vs. Placebo | 60 | 115 | 1 | 56 | 29.22 [4.16,205.41] | 50.3 | 0.01# | |
| Total (95% CI) | 89 | 188 | 2 | 93 | 22.01 [5.53,87.60] | 100.0 | 0 | 0.62 |
| CDLQI 0/1 | ||||||||
| Ustekinumab vs. Placebo | 29 | 73 | 4 | 37 | 3.67 [1.40,9.67] | 22.6 | 0.01# | |
| Ixekizumab vs. Placebo | 74 | 115 | 13 | 56 | 2.77 [1.69,4.55] | 37.6 | 0.01# | |
| Secukinumab vs. Etanercept | 42 | 80 | 16 | 41 | 1.35 [0.87,2.08] | 39.8 | 0.18 | |
| Total (95% CI) | 145 | 268 | 33 | 134 | 2.22 [1.19,4.14] | 100.0 | 71 | 0.03* |
CI, confidence interval; PASI, Psoriasis Area and Severity Index; PASI75, 75% improvement in the PASI from baseline; PASI50, 50% improvement in the PASI from baseline; PASI90, 90% improvement in the PASI from baseline; PASI100, 100% improvement in the PASI from baseline; sPGA, static Physician’s Global Assessment of Disease; sPGA 0/1, the score of static Physician’s Global Assessment of Disease achieves 0 or 1; sPGA 0, the score of static Physician’s Global Assessment of Disease achieves 0; CDLQI, Children’s Dermatology Life Quality Index; CDLQI 0/1, Children’s Dermatology Life Quality Index achieves 0 or 1; MTX, methotrexate. *p < 0.05, #p < 0.0001.
PASI 50
Two included articles employed PASI 50 as the outcome indicator, and the response rate with biologics was better than that with placebo at the endpoint ( Table 2 , Data Sheet 2 ; P-value = 0.27, RR = 2.83, 95%[CI] [2.20, 3.63]).
PASI 90
Compared with placebo treatment, ustekinumab, ixekizumab, and etanercept afforded 10.64-, 14.61-, and 4.1-fold better outcomes (P-value < 0.01), respectively, and the results further showed that secukinumab may be better than etanercept ( Table 2 , Data Sheet 2 ; P-value < 0.01, RR = 1.56, 95%[CI] [1.14, 2.15]). In summary, the PASI 90 of the biologics group was 3.85-fold higher than that of the control group ( Table 2 , Data Sheet 2 ; P-value < 0.01, RR = 3.85, 95%[CI] [1.40, 10.58]).
PASI 100
The PASI 100 score after ixekizumab treatment was markedly greater (27.76-fold better) than that after placebo treatment ( Table 2 ; Data Sheet 2 ; P-value < 0.01, RR = 27.76, 95%[CI] [3.94, 195.31]), and adalimumab was 5.29-fold better than MTX treatment ( Table 2 , Data Sheet 2 ; P-value < 0.10, RR = 5.29, 95%[CI] [0.71, 39.42]). The PASI 100 response rate with biologics was 5.89-fold higher than that in the control group ( Table 2 , Data Sheet 2 ; P-value < 0.01, RR = 5.89, 95%[CI] [0.84, 41.51]).
Static Physician’s Global Assessment (sPGA)
The sPGA score assesses the average thickness, erythema, and scaling of psoriatic lesions, with scores ranging from 0 (clear) to 4 (severe), with 0/1 indicating clear or almost clear. Two studies used sPGA 0 as the outcome indicator, and the results were significant, favoring treatment with ustekinumab and ixekizumab over placebo ( Table 2 ; Data Sheet 2 ; P-value = 0.62, RR = 22.01, 95%[CI] [5.53, 87.60]). Additionally, four reports used sPGA 0/1 as the evaluation indicator, demonstrating better efficacy following treatment with biologics ( Table 2 , Data Sheet 2 ; P-value < 0.01, RR = 3.15, 95%[CI] [1.26, 7.86]).
Children’s Dermatology Life Quality Index (CDLQI)
Three studies used the CDLQI scores to evaluate the general curative effect as a complete response. The biologics (ustekinumab and ixekizumab) showed better performance against psoriatic pathogenesis in adolescent patients ( Table 2 , Data Sheet 2 ). Overall, the efficacy outcomes were better in the biologic group than in the control group.
Safety
AEs
We compared among the groups. Surprisingly, there was no significant difference in the frequency of overall adverse reactions between the biologic and control groups. ( Table 3 , Data Sheet 3 ; P-value = 0.53, RR = 1.05, 95%[CI] [0.92, 1.19]) and fewer AEs were observed in the ustekinumab group than in the placebo group (P-value = 0.37, RR = 0.84, 95%[CI] [0.58, 1.22]).
Table 3.
Adverse events and serious adverse events reported for biological agents used in pediatric patients with psoriasis.
| Subgroup | Experimental | Control | Risk ratio M-H (95% CI) | Study weight, % | I2, % | P-value | ||
|---|---|---|---|---|---|---|---|---|
| Events | Total | Events | Total | |||||
| ALL AEs | ||||||||
| Ustekinumab vs. Placebo | 35 | 73 | 21 | 37 | 0.84 [0.58,1.22] | 15.8 | 0.37 | |
| Ixekizumab vs. Placebo | 64 | 115 | 25 | 56 | 1.25 [0.89,1.74] | 19.1 | 0.20 | |
| Etanercept vs. Placebo | 914 | 106 | 144 | 105 | 1.44 [0.81,1.60] | 18.0 | 0.44 | |
| Secukinumab vs. Etanercept | 68 | 80 | 34 | 41 | 1.02 [0.87,1.21] | 25.5 | 0.77 | |
| Adalimumab vs. MTX | 56 | 77 | 28 | 37 | 0.96 [0.76,1.21] | 21.5 | 0.73 | |
| Total (95% CI) | 1173 | 519 | 284 | 345 | 1.05 [0.92,1.19] | 100.0 | 0 | 0.53 |
| Serious AEs | ||||||||
| Ustekinumab vs. Placebo | 1 | 73 | 0 | 37 | 1.54 [0.06,36.92] | 4.8 | 0.79 | |
| Ixekizumab vs. Placebo | 1 | 115 | 0 | 56 | 1.47 [0.06,35.62] | 4.9 | 0.81 | |
| Etanercept vs. Placebo | 4 | 106 | 3 | 105 | 1.32 [0.30,5.76] | 22.1 | 0.71 | |
| Secukinumab vs. Etanercept | 7 | 80 | 5 | 41 | 0.72 [0.24,2.12] | 48.4 | 0.55 | |
| Adalimumab vs. MTX Total (95% CI) |
9 22 |
77 451 |
2 10 |
37 276 |
2.16 [0.49,9.51] 1.21 [0.60,2.44] |
19.8 100.0 |
0 | 0.31 0.82 |
CI, confidence interval; MTX, methotrexate; AEs, Adverse Events. *p < 0.05, #p < 0.000.
Serious AEs
We also compared the frequency of serious adverse reactions between the biologics and placebo and MTX groups. The probability of severe AEs was higher in the biologic group than in the control group ( Table 3 , Data Sheet 4 ; P-value = 0.82, RR = 1.21, 95%[CI] [0.60, 2.44]).
Specific adverse reactions
Type α AEs occur after the abundant release of inflammatory factors; these AEs are most frequent with complicated and changeable symptoms. Type β AEs are immune-mediated and are more serious than type α AEs, including immediate and delayed hypersensitivity reactions. Types γ and δ AEs are short- and long-term toxicities, respectively. Type ϵ AEs occur during drug withdrawal, particularly when a drug is suddenly stopped (14).
Type α
In this study, type α AEs were the most frequently observed adverse reactions. The incidence of infection was 1.06-fold higher in the biologics group than in the control group (P-value = 0.48; RR = 1.06; 95%[CI] [0.87, 1.31]) ( Table 4 , Data Sheet 5 ). The occurrence of gastrointestinal infections was 0.93-fold lower in the biologic group than in the control group (P-value = 0.77; RR = 0.93; 95%[CI] [0.55, 1.59]). Furthermore, the probability of headaches was 2.65-fold greater in the biologic group than in the control group (P-value = 0.45; RR = 2.65; 95%[CI] [1.77, 3.98]). Serious infection in the biological group was 3.81-fold higher than that in the placebo treatment group (P-value = 0.48; RR = 3.81; 95%[CI] [0.49, 29.51]), for which no serious infection was reported. Other frequent AEs included cough, influenza, nausea, upper respiratory tract infection, Candida infection, cytopenia, neutropenia, vomiting, streptococcal pharyngitis, pharyngolaryngeal pain, and rhinitis ( Figure 3 ).
Table 4.
Specific adverse events reported on using biological agents in pediatric patients with psoriasis.
| Subgroup | Experimental | Control | Risk ratio M-H(95% CI) | Study weight, % | I2, % | P-value | ||
|---|---|---|---|---|---|---|---|---|
| Events | Total | Events | Total | |||||
| Infection | ||||||||
| Etanercept vs. Placebo | 37 | 115 | 14 | 56 | 1.29 [0.76,2.18] | 22.7 | 0.35 | |
| Secukinumab vs. Etanercept | 57 | 80 | 27 | 41 | 1.08 [0.83,1.40] | 43.1 | 0.55 | |
| Adalimumab vs. MTX | 39 | 77 | 21 | 37 | 0.89 [0.62,1.28] | 34.2 | 0.53 | |
| Total (95% CI) | 133 | 272 | 62 | 134 | 1.06 [0.87,1.31] | 100.0 | 0 | 0.48 |
| Gastrointestinal infection | ||||||||
| Secukinumab vs. Etanercept | 25 | 80 | 14 | 41 | 0.92 [0.54,1.56] | 96.5 | 0.75 | |
| Adalimumab vs. MTX | 1 | 77 | 0 | 37 | 1.46 [0.06,35.04] | 3.5 | 0.81 | |
| Total (95% CI) | 26 | 157 | 14 | 78 | 0.93 [0.55,1.59] | 100.0 | 0 | 0.77 |
| Headache | ||||||||
| Etanercept vs. Placebo | 60 | 174 | 20 | 174 | 2.98 [1.91,4.64] | 79.1 | 0.98 | |
| Secukinumab vs. Etanercept | 11 | 80 | 4 | 41 | 1.41 [0.48,4.15] | 20.9 | 0.53 | |
| Total (95% CI) | 71 | 254 | 24 | 215 | 2.65 [1.77,3.98] | 100.0 | 0 | 0.45 |
| Serious Infections | ||||||||
| Ixekizumab vs. Placebo | 1 | 115 | 0 | 56 | 1.47 [0.06,35.62] | 57.2 | 0.81 | |
| Etanercept vs. Placebo | 3 | 106 | 0 | 105 | 6.93 [0.36,132.62] | 42.8 | 0.20 | |
| Total (95% CI) | 4 | 221 | 0 | 161 | 3.81 [0.49,29.51] | 100.0 | 0 | 0.48 |
| Hypersensitivity | ||||||||
| Ixekizumab vs. Placebo | 6 | 115 | 1 | 56 | 2.92 [0.36,23.69] | 12.6 | 0.32 | |
| Secukinumab vs. Etanercept | 12 | 80 | 5 | 41 | 1.23 [0.46,3.25] | 62.0 | 0.68 | |
| Adalimumab vs. MTX | 1 | 77 | 2 | 37 | 0.24 [0.02,2.57] | 25.3 | 0.24 | |
| Total (95% CI) | 19 | 272 | 8 | 134 | 1.19 [0.54,2.26] | 100.0 | 19 | 0.29 |
| Injection-site reactions | ||||||||
| Ixekizumab vs. Placebo | 14 | 115 | 1 | 56 | 6.82 [0.92,50.55] | 17.3 | 0.06 | |
| Etanercept vs. Placebo | 63 | 174 | 6 | 174 | 5.02 [0.48,52.83] | 37.8 | 65 | 0.18 |
| Secukinumab vs. Etanercept | 7 | 80 | 4 | 41 | 0.90 [0.28,2.89] | 22.9 | 0.86 | |
| Adalimumab vs. MTX | 7 | 77 | 3 | 37 | 1.12 [0.31,4.09] | 22.1 | 0.86 | |
| Total (95% CI) | 91 | 446 | 14 | 308 | 2.60 [0.66,10.25] | 100.0 | 79 | 0.01 |
| Nasopharyngitis | ||||||||
| Etanercept vs. Placebo | 59 | 174 | 12 | 174 | 5.00 [2.89,8.67] | 64.1 | 0.62 | |
| Secukinumab vs. Etanercept | 28 | 80 | 11 | 41 | 1.30 [0.73,2.35] | 38.6 | 0.37 | |
| Total (95% CI) | 87 | 254 | 23 | 215 | 2.80 [0.95,8.19] | 100.0 | 82 | 0.01# |
| Hand fracture | ||||||||
| Ustekinumab vs. Placebo | 20 | 73 | 14 | 37 | 0.72 [0.41,1.26] | 96.5 | 0.26 | |
| Adalimumab vs. MTX | 1 | 77 | 0 | 37 | 1.46 [0.06,35.04] | 3.5 | 0.81 | |
| Total (95% CI) | 21 | 150 | 14 | 74 | 0.75 [0.43,1.30] | 100.0 | 0 | 0.67 |
| Skin eruption | ||||||||
| Etanercept vs. Placebo | 16 | 106 | 0 | 105 | 32.69 [1.99,537.95] | 44.0 | 0.01# | |
| Secukinumab vs. Etanercept | 24 | 80 | 10 | 41 | 1.23 [0.65,2.32] | 56.0 | 0.52 | |
| Total (95% CI) | 40 | 186 | 10 | 146 | 5.21 [0.10,260.11] | 100.0 | 87 | 0.01# |
CI, confidence interval; MTX, methotrexate; AEs, adverse events;*p < 0.05, #p < 0.00 01.
Figure 3.
Mapping of specific adverse events between biological agents and control groups. MTX: methotrexate. IBD: inflammatory bowel disease.
Type β
In the present study, injection-site reactions were the most common type of β-adverse reactions, and the incidence of infection at the injection site was 2.60-fold higher in the biologics group than in the control group (P-value = 0.01; RR = 2.60; 95%[CI] [0.66, 10.25]) ( Table 4 , Data Sheet 5 ). Furthermore, the biologics group presented a 1.19-fold higher probability of hypersensitivity reactions than the control group (P-value = 0.29; RR = 1.19; 95%[CI] [0.54, 2.62]). Nasal congestion is another commonly reported type of β AE ( Figure 3 ).
Type γ
The most frequently reported type γ adverse reaction was a hand fracture. The probability of hand fracture was 0.75-fold higher in the biologic group than in the control group (P-value = 0.67; RR = 0.75; 95%[CI] [0.43, 1.30]) ( Table 4 , Data Sheet 5 ). Other common type γ AEs are Crohn’s disease (CD) and irritable bowel disease; only one case of these two reactions, induced following ixekizumab therapy, was reported in the present study ( Figure 3 ).
Type δ
Skin eruption was the only type δ adverse reaction reported, for which the biologic group presented a 5.21-fold higher probability than the control group (P-value = 0.006; RR = 5.21; 95%[CI] [0.10, 260.11]) ( Table 4 , Data Sheet 5 ) ( Figure 3 ).
Type ϵ
Three types of adverse reactions, namely, depression, agitation, and self-injury, were documented in the biologics group ( Figure 3 ).
Bayesian analysis outcomes
Five RCTs involving 791 patients reported a PASI of 75. The Bayesian analysis performed was a network meta-analysis ( Figure 4 ). The size of the nodes represented the sample size of each study; the link between two nodes indicated direct comparisons, with the thickness of the lines representing the number of reports. No inconsistencies were detected when using the node-splitting method (P-value > 0.05) ( Data Sheet 6 ). The scatter chart revealed a low bias ( Figure 5 ).
Figure 4.
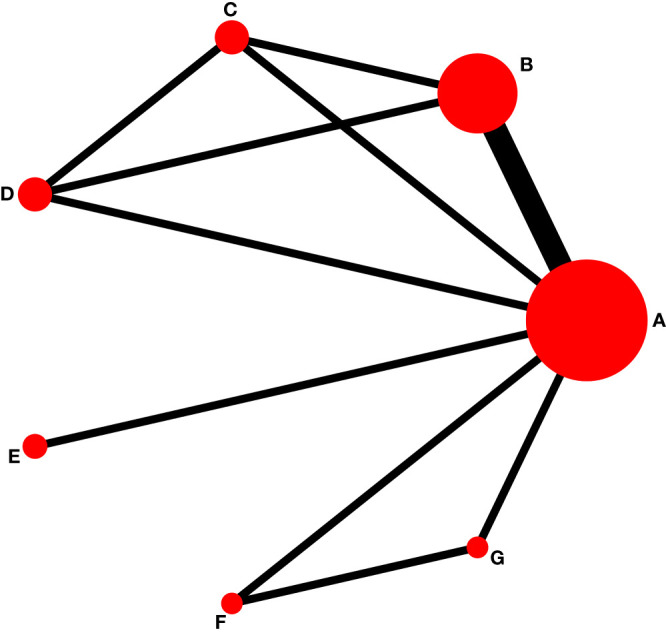
Network data on biologic agents in the treatment of psoriasis in children. LD, Low dose; HD, High dose. (A) placebo; (B) TNF-α Etanercept; (C) IL-17A Secukinumab LD; (D) IL-17A Secukinumab HD; (E) IL-17A Ixekizumab; (F) IL-23 Ustekinumab LD; (G) IL-23 Ustekinumab HD.
Figure 5.
Comparison-adjusted funnel plot. A: placebo; B: TNF-α Etanercept; C: IL-17A Secukinumab LD; D: IL-17A Secukinumab HD; E: IL-17A Ixekizumab; F: IL-23 Ustekinumab LD; G: IL-23 Ustekinumab HD.
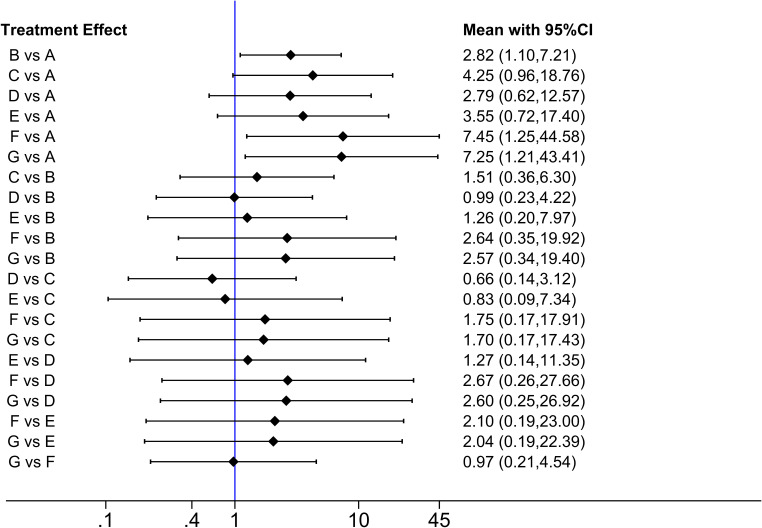
Based on the Bayesian framework, we performed a meta-analysis using an MCMC random-effects model and generated 21 pairs of comparisons, of which three showed significant differences ( Figure 6 ). Compared with that of A (placebo), the efficacies of B (TNF-α etanercept) (RR: 2.82, 95%[CI] [1.10,7.21]), F (IL-23 ustekinumab LD) (RR: 7.45, 95%[CI] [1.25,44.58]), and G (IL-23 ustekinumab HD) (RR: 7.25, 95%[CI] [1.21,43.41]) were superior. There were no other statistically significant differences.
Figure 6.
Meta-analysis forest plot. A: placebo; B: TNF-α Etanercept; C: IL-17A Secukinumab LD; D: IL-17A Secukinumab HD; E: IL-17A Ixekizumab; F: IL-23 Ustekinumab LD; G: IL-23 Ustekinumab HD.
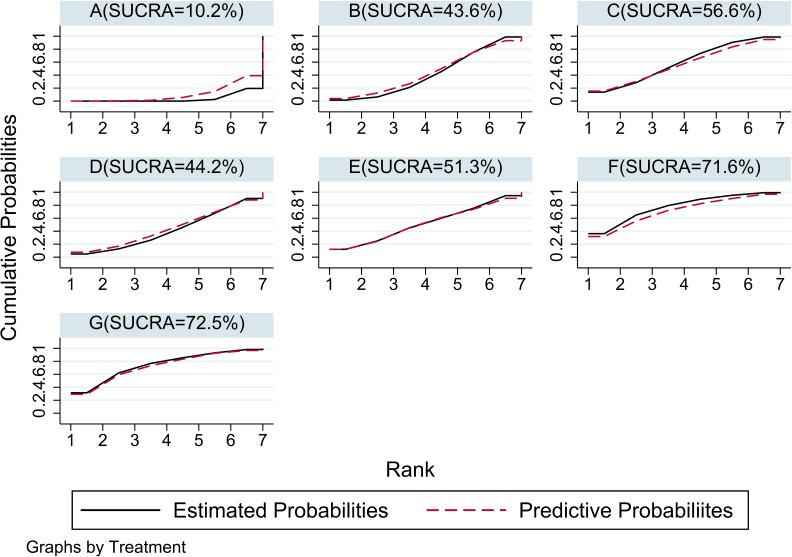
For the use of biologics in pediatric psoriasis patients, in this analysis, IL-23 ustekinumab HD(G) had the best efficacy (SUCRA = 72.5%), followed by IL-23 ustekinumab LD(F) (SUCRA = 71.6%)IL-17A Secukinumab (C) (SUCRA = 56.6%), IL-17A Ixekizumab (E) (SUCRA = 51.3%), IL-17A Secukinumab HD (D) (SUCRA = 44.2%), TNF-α Etanercept (B) (SUCRA = 43.6%), and placebo (A) (SUCRA = 10.2%). The SUCRA grade chart is shown in Figure 7 . The efficacy comparison estimated using the ladder diagram is presented in Table 5 . Bolded font indicates a statistical difference.
Figure 7.
SUCRA rank plot of seven interventions. If the SUCRA approaches 100%, the intervention is the best among the included trials; conversely, if it is close to 0%, it represents the worst intervention. (A) placebo; (B) TNF-α Etanercept; (C) IL-17A Secukinumab LD; (D) IL-17A Secukinumab HD; (E) IL-17A Ixekizumab; (F) IL-23 Ustekinumab LD; (G) IL-23 Ustekinumab HD.
Table 5.
Efficacy comparison of 7 interventions (random-effects model of MCMC method).
| G | 1.03 (0.22,4.80) | 0.49 (0.04,5.36) | 0.38 (0.04,3.99) | 0.59 (0.06,6.00) | 0.39 (0.05,2.94) | 0.14 (0.02,0.83) |
| 0.97 (0.21,4.54) | F | 0.48 (0.04,5.21) | 0.37 (0.04,3.88) | 0.57 (0.06,5.83) | 0.38 (0.05,2.85) | 0.13 (0.02,0.80) |
| 2.04 (0.19,22.39) | 2.10 (0.19,23.00) | E | 0.79 (0.09,7.03) | 1.20 (0.14,10.55) | 0.79 (0.13,5.04) | 0.28 (0.06,1.38) |
| 2.60 (0.25,26.92) | 2.67 (0.26,27.66) | 1.27 (0.14,11.35) | D | 1.52 (0.32,7.24) | 1.01 (0.24,4.31) | 0.36 (0.08,1.61) |
| 1.70 (0.17,17.43) | 1.75 (0.17,17.91) | 0.83 (0.09,7.34) | 0.66 (0.14,3.12) | C | 0.66 (0.16,2.77) | 0.24 (0.05,1.04) |
| 2.57 (0.34,19.40) | 2.64 (0.35,19.92) | 1.26 (0.20,7.97) | 0.99 (0.23,4.22) | 1.51 (0.36,6.30) | B | 0.35 (0.14,0.91) |
| 7.25 (1.21,43.41) | 7.45 (1.25,44.58) | 3.55 (0.72,17.40) | 2.79 (0.62,12.57) | 4.25 (0.96,18.76) | 2.82 (1.10,7.21) | A |
A: placebo; B: TNF-α Etanercept; C, IL-17A Secukinumab LD; D, IL-17A Secukinumab HD; E, IL-17A Ixekizumab; F, IL-23 Ustekinumab LD; G, IL-23 Ustekinumab HD.Bolded font indicates a statistical difference.
Discussion
Juvenile-onset psoriasis is typically associated with a high probability of a positive family history and more serious symptoms, necessitating prompt therapeutic intervention (21). Treatment with biologics is the most effective for psoriasis, with a low incidence of adverse reactions in adults and a certain degree of safety (22). However, caution should be exercised when treating special populations, such as children. The present analysis provides comprehensive data on the use of biologics in children and adolescents with psoriasis. This is the first report of AEs in pediatric patients with psoriasis who were systematically exposed to biologics.
Studies have reported that the curative effect of biologics is considerable in pediatric patients compared to that in controls, and the AEs rates are similar to those in adults. In the present study, accumulated data suggested that anti-TNF-α preparations, such as etanercept, have lower efficacy, while adalimumab seemed more effective in treating psoriasis in children and adolescents. A previous study confirmed that the efficacy of etanercept may be similar to that of MTX (23, 24), which is consistent with our findings. Our Bayesian analysis showed differences in the efficacy of etanercept TNF-α inhibitor and ustekinumab IL-23 inhibitor compared with placebo. The highest dose of ustekinumab showed the best efficacy. Based on subjective evaluation scales (sPGA and CDLQI), ixekizumab was preferred for symptom improvement by clinicians, whereas ustekinumab was preferred by patients. The frequency of AEs between the biologics group and controls was almost equivalent, whereas the symptom severity was 1.22-fold higher in the biologics group than in the control group. These results indicated that the probability of AEs following treatment with biologics was not high, but rather more severe, which may decrease their acceptability.
To characterize the severe AEs caused by biologics, we classified and analyzed the different AEs induced by the biological agents. Accordingly, type α adverse reactions appeared to occur the most frequently. Children with psoriasis are prone to infections, headache, nasopharyngitis, injection-site infections, and skin eruptions. Compared to other biologics, etanercept is more likely to cause adverse reactions. Ustekinumab caused fewer AEs in children with psoriasis and could be recommended as a therapeutic option. In summary, our analysis showed that ustekinumab conferred better clinical efficacy with a low incidence of AEs, and hence could be recommended.
Herein, we reviewed retrospective RCTs evaluating biologics to determine cautionary issues and treatment risks during therapy in adolescents. This information could potentially enhance the quality and efficiency of future clinical research. The strengths of this study are as follows: (i) the included studies were of high quality, minimizing selection bias; (ii) all included studies had corresponding control groups, monitoring the bias and generating compelling evidence; and (iii) no pharmaceutical industry was involved in the present work. However, the analysis was limited by the small sample size, which was insufficient to determine the recommended dose and treatment duration of novel biological agents for pediatric patients. Future studies should focus on applying biologics in special populations, and clinicians should encourage relevant patients to enroll in prospective pharmacovigilance registries, undoubtedly helping to tackle unresolved questions in this field.
Finally, because of the short duration of use of biologics in children and the small number of relevant RCTs, the sample size of this study was not large enough, and the database search only yielded studies on secukinumab, ixekizumab, adalimumab, ustekinumab, and etanercept; the other biologics were not evaluated. Furthermore, the treatment duration of the included studies was not uniform and the recurrence rate was not reported.
Conclusion
In conclusion, biologics can effectively treat children with psoriasis and can greatly improve their quality of life, eliminate lesions, and improve pruritus severity. Although AEs were reported in all the included studies, biologics can still be safely used to treat pediatric patients with moderate-to-severe psoriasis. Additionally, our analysis revealed that ustekinumab conferred good clinical efficacy with a low incidence of AEs, and hence could be recommended.
Data availability statement
The original contributions presented in the study are included in the article/ Supplementary Material . Further inquiries can be directed to the corresponding authors.
Author contributions
XC and YR conceived the study. XC and YR designed this study. Y-qZ, YL, and JC performed literature search and data extraction. XC and MZ assessed the quality of the trials and analyzed the data. YR and CW drafted the original manuscript, and BL and XL contributed to the manuscript revision. All authors have read and approved the final manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (grant nos. 81874470 and 82074427), National Key Research and Development Program of China (grant no. 2018YFC1705302), Xinglin Scholar, Shanghai University of Traditional Chinese Medicine (No. RY411.14.12), Shanghai Pujiang Talent Program (No. 2020PJD067), Science and Technology Commission of Shanghai Municipality (Nos. 21Y21920100, 21Y21920102), and Shanghai Municipal Commission of Economy and Information Technology (No. 2020-RGZN-02038).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2022.896550/full#supplementary-material
Abbreviations
TH1, T helper type 1 cells; TH17, T helper type 17 cells; TNF-α, tumor necrosis factor-α; IL-23, interleukin-23; IL-17, interleukin-17; IL-12, interleukin-12; PRISMA, Preferred Reporting Items for Systematic Review and Meta-Analysis; RCTs, randomized control trials; AEs, adverse events; PASI 75, Psoriasis Area and Severity Index 75; PASI 50/75/90/100, PASI score decreases more than 50%/75%/90%/100% from baseline; sPGA, Static Physician’s Global Assessment; sPGA0/1, Static Physician’s Global Assessment achieve 0 or 1; sPGA0, Static Physician’s Global Assessment achieved 0; CDQLI, Children’s Dermatology Life Quality Index; DLQI 0/1, Children’s Dermatology Life Quality Index achieve 0 or 1; RR, Risk ratios; CI, confidence intervals; MD, mean difference; SMD, standard mean difference; MTX, methotrexate; LD, Low dose; HD, High dose.
References
- 1. Michalek IM, Loring B, John SM. A systematic review of worldwide epidemiology of psoriasis. J Eur Acad Dermatol Venereol JEADV (2017) 31(2):205–12. doi: 10.1111/jdv.13854 [DOI] [PubMed] [Google Scholar]
- 2. Raychaudhuri SP, Gross J. A comparative study of pediatric onset psoriasis with adult onset psoriasis. Pediatr Dermatol (2000) 17(3):174–8. doi: 10.1046/j.1525-1470.2000.01746.x [DOI] [PubMed] [Google Scholar]
- 3. Bronckers I, Paller AS, West DP, Lara-Corrales I, Tollefson MM, Tom WL, et al. A comparison of psoriasis severity in pediatric patients treated with methotrexate vs biologic agents. JAMA Dermatol (2020) 156(4):384–92. doi: 10.1001/jamadermatol.2019.4835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Armstrong AW, Read C. Pathophysiology, clinical presentation, and treatment of psoriasis: A review. Jama (2020) 323(19):1945–60. doi: 10.1001/jama.2020.4006 [DOI] [PubMed] [Google Scholar]
- 5. Yiu ZZN, Exton LS, Jabbar-Lopez Z, Mohd Mustapa MF, Samarasekera EJ, Burden AD, et al. Risk of serious infections in patients with psoriasis on biologic therapies: A systematic review and meta-analysis. J Invest Dermatol (2016) 136(8):1584–91. doi: 10.1016/j.jid.2016.03.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Paller AS, Siegfried EC, Eichenfield LF, Pariser D, Langley RG, Creamer K, et al. Long-term etanercept in pediatric patients with plaque psoriasis. J Am Acad Dermatol (2010) 63(5):762–8. doi: 10.1016/j.jaad.2010.04.004 [DOI] [PubMed] [Google Scholar]
- 7. Menter A, Strober BE, Kaplan DH, Kivelevitch D, Prater EF, Stoff B, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol (2019) 80(4):1029–72. doi: 10.1016/j.jaad.2018.11.057 [DOI] [PubMed] [Google Scholar]
- 8. Wang Q, Li M, Hu X, Luo Q, Hao P. Autologous blood or autologous serum acupoint injection therapy for psoriasis vulgaris: A protocol for a systematic review and meta-analysis. Med (Baltimore) (2020) 99(23):e20555. doi: 10.1097/md.0000000000020555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Plavec TV, Kuchař M, Benko A, Lišková V, Černý J, Berlec A, et al. Engineered lactococcus lactis secreting IL-23 receptor-targeted REX protein blockers for modulation of IL-23/Th17-Mediated inflammation. Microorganisms (2019) 7(5):152. doi: 10.3390/microorganisms7050152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ollech A, Zvulunov A, Pavlovsky L, Hodak E, Ben-Amitai D. Biologic treatment of recalcitrant pediatric psoriasis: a case series from a tertiary medical center. J Dermatol Treat (2019) 30(2):152–5. doi: 10.1080/09546634.2018.1476655 [DOI] [PubMed] [Google Scholar]
- 11. Kimball AB, Guenther L, Kalia S, de Jong E, Lafferty KP, Chen DY, et al. Pregnancy outcomes in women with moderate-to-Severe psoriasis from the psoriasis longitudinal assessment and registry (PSOLAR). JAMA Dermatol (2021) 157(3):301–6. doi: 10.1001/jamadermatol.2020.5595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Körber A, Papavassilis C, Bhosekar V, Reinhardt M. Efficacy and safety of secukinumab in elderly subjects with moderate to severe plaque psoriasis: A pooled analysis of phase III studies. Drugs Aging (2018) 35(2):135–44. doi: 10.1007/s40266-018-0520-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ (Clin Res ed) (2009) 339:b2700. doi: 10.1136/bmj.b2700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pichler WJ. Adverse side-effects to biological agents. Allergy (2006) 61(8):912–20. doi: 10.1111/j.1398-9995.2006.01058.x [DOI] [PubMed] [Google Scholar]
- 15. Bodemer C, Kaszuba A, Kingo K, Tsianakas A, Morita A, Rivas E, et al. Secukinumab demonstrates high efficacy and a favourable safety profile in paediatric patients with severe chronic plaque psoriasis: 52-week results from a phase 3 double-blind randomized, controlled trial. J Eur Acad Dermatol Venereol JEADV (2021) 35(4):938–47. doi: 10.1111/jdv.17002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Landells I, Marano C, Hsu M-C, Li S, Zhu Y, Eichenfield LF, et al. Ustekinumab in adolescent patients age 12 to 17 years with moderate-to-severe plaque psoriasis: Results of the randomized phase 3 CADMUS study. J Am Acad Dermatol (2015) 73(4):594–603. doi: 10.1016/j.jaad.2015.07.002 [DOI] [PubMed] [Google Scholar]
- 17. Papp K, Thaçi D, Marcoux D, Weibel L, Philipp S, Ghislain PD, et al. Efficacy and safety of adalimumab every other week versus methotrexate once weekly in children and adolescents with severe chronic plaque psoriasis: a randomised, double-blind, phase 3 trial. Lancet (2017) 390(10089):40–9. doi: 10.1016/s0140-6736(17)31189-3 [DOI] [PubMed] [Google Scholar]
- 18. Paller AS, Seyger MMB, Alejandro Magarinos G, Bagel J, Pinter A, Cather J, et al. Efficacy and safety of ixekizumab in a phaseIII, randomized, double-blind, placebo-controlled study in paediatric patients with moderate-to-severe plaque psoriasis (IXORA-PEDS). Br J Dermatol (2020) 183(2):231–41. doi: 10.1111/bjd.19147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Paller AS, Siegfried EC, Langley RG, Gottlieb AB, Pariser D, Landells I, et al. Etanercept treatment for children and adolescents with plaque psoriasis. New Engl J Med (2008) 358(3):241–51. doi: 10.1056/NEJMoa066886 [DOI] [PubMed] [Google Scholar]
- 20. Siegfried EC, Eichenfield LF, Paller AS, Pariser D, Creamer K, Kricorian G. Intermittent etanercept therapy in pediatric patients with psoriasis. J Am Acad Dermatol (2010) 63(5):769–74. doi: 10.1016/j.jaad.2009.10.046 [DOI] [PubMed] [Google Scholar]
- 21. Galili E, Barzilai A, Shreberk-Hassidim R, Merdler I, Caspi T, Astman N. Neuropsychiatric comorbidity among adolescents with psoriasis. Br J Dermatol (2018) 178(4):910–6. doi: 10.1111/bjd.16031 [DOI] [PubMed] [Google Scholar]
- 22. Lu W, Deng Y, Fang Z, Zhai Q, Cui S, Zhao J, et al. Potential role of probiotics in ameliorating psoriasis by modulating gut microbiota in imiquimod-induced psoriasis-like mice. Nutrients (2021) 13(6):2010. doi: 10.3390/nu13062010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Egeberg A, Ottosen MB, Gniadecki R, Broesby-Olsen S, Dam TN, Bryld LE, et al. Safety, efficacy and drug survival of biologics and biosimilars for moderate-to-severe plaque psoriasis. Br J Dermatol (2018) 178(2):509–19. doi: 10.1111/bjd.16102 [DOI] [PubMed] [Google Scholar]
- 24. Jabbar-Lopez ZK, Yiu ZZN, Ward V, Exton LS, Mohd Mustapa MF, Samarasekera E, et al. Quantitative evaluation of biologic therapy options for psoriasis: A systematic review and network meta-analysis. J Invest Dermatol (2017) 137(8):1646–54. doi: 10.1016/j.jid.2017.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/ Supplementary Material . Further inquiries can be directed to the corresponding authors.