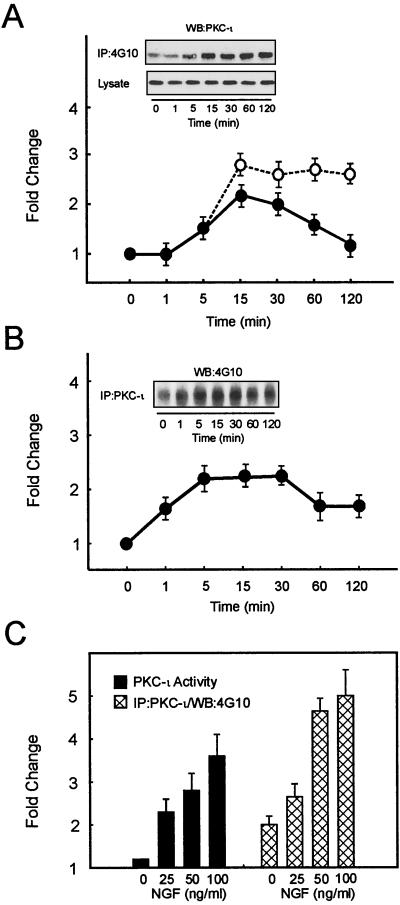
FIG. 1.
NGF treatment of PC12 cells increases activation and tyrosine phosphorylation of PKC-ι. An equivalent amount of protein (500 μg) was used to determine PKC-ι activity by immune complex kinase assay, and the tyrosine phosphorylation of PKC-ι was examined by immunoprecipitation of PKC-ι followed by blotting with the 4G10 antiphosphotyrosine (anti-pTyr) antibody or immunoprecipitation (IP) of proteins containing phosphotyrosine and Western blotting with the PKC-ι antibody. The autoradiographs were scanned densitometrically and plotted to show the fold change. (A) PC12 cells were stimulated with NGF (100 ng/ml) for the indicated times. PKC-ι activity (●) and tyrosine phosphorylation of PKC-ι (○) were determined. Lysate used for immunoprecipitation was also probed with anti-PKC-ι. PKC-ι Western blots of the immunoprecipitate and the lysate are shown as insets. (B) PC12 cells were stimulated with NGF (50 ng/ml) and immunoprecipitated with the PKC-ι antibody, followed by blotting with the 4G10 anti-pTyr antibody. (C) PC12 cells were stimulated with various doses of NGF as shown for 15 min. PKC-ι activity was examined. The tyrosine phosphorylation of PKC-ι was examined by immunoprecipitation of PKC-ι followed by blotting with the 4G10 anti-pTyr antibody. The results (means ± SEM) are representative of three other independent experiments.