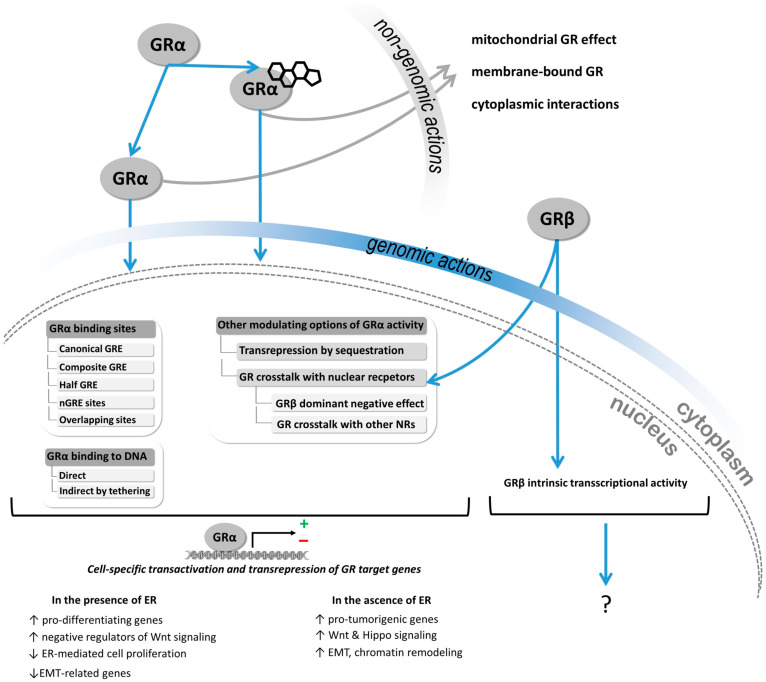
Figure 1.
Mechanism of action of glucocorticoid receptor alpha (GRα) and beta (GRβ) in breast cancer cell. GR activity is strongly context-dependent, and determined by, among others, GR expression, splicing resulting in splice isoforms, posttranslational modifications and nuclear receptor crosstalk [8,9,10,23,24]. GRα activation can be ligand-dependent or -independent. By translocating into the nucleus, it binds to specific regulatory parts of the DNA (GR responsive elements, GREs) through which several genes’ expressions are induced or repressed in a cell type-specific manner. GRα and ER coactivation enhanced GRα binding to both GRE and oestrogen-responsive element (ERE), leading to an increased expression of pro-differentiating genes and negative regulators of pro-oncogenic Wnt signaling, and a decreased expression of epithelial–mesenchymal transition (EMT)-related genes. However, in the absence of ER, ligand-bound GRα binds to the GREs of several pro-tumourigenic genes, driving drug resistance and progression in TNBC (see details in the text, and in [6,23]). GRβ, due to its shorter sequence, cannot bind the ligand, but it is able to form a heterodimer with GRα. By binding to GREs, GRβ impairs GRα-mediated genomic actions, which is called the dominant–negative effect. While it has been described that GRβ is able to regulate proliferation and migration in other cell types, there is no clear evidence for its role in breast cancer development and progression (see details in the text, and in [22]).