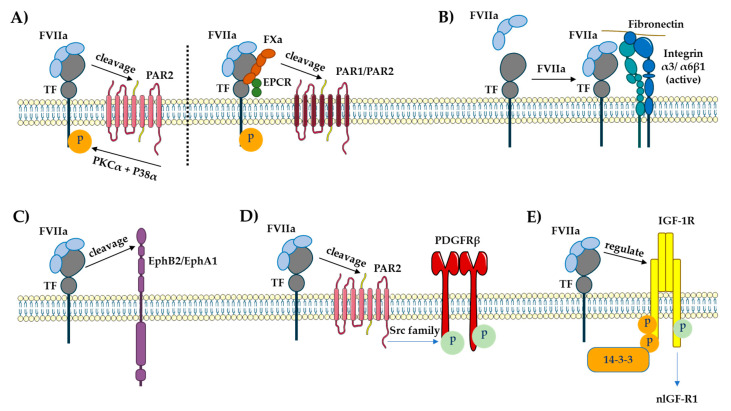
Figure 2.
Cell surface-bound full-length TF interacts with a number of different proteins in order to initiate signaling and achieve specificity. (A) PAR1 is cleaved by the TF/FVIIa/FXa complex whereas PAR2 is cleaved by both TF/FVIIa and TF/FVIIa/FXa. TF cytoplasmic domain is Ser253 phosphorylated by PKCα and Ser258 phosphorylated by P38α. (B) Association of TF with β1 integrins is regulated by TF extracellular ligand binding and independent of PAR2 signaling or proteolytic activity of VIIa. (C) EphB2 and EphA2 are proteolytic targets of TF/FVIIa. (D) TF/FVIIa transactivates the PDGFRβ in a PAR2 and Src family. (E) TF/FVIIa transactivates IGF-1R and mediates the signaling cascades generated by IGF-1 stimulation. Parts of the figure were drawn using elements from Servier Medical Art. Servier Medical Art by Servier is licensed under a Creative Commons Attribution 3.0 Unported License (https://creativecommons.org/licenses/by/3.0/) (accessed on 16 May 2022).