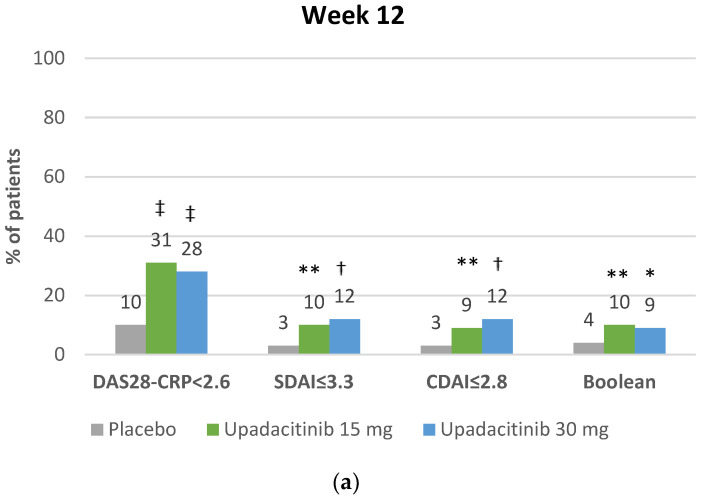
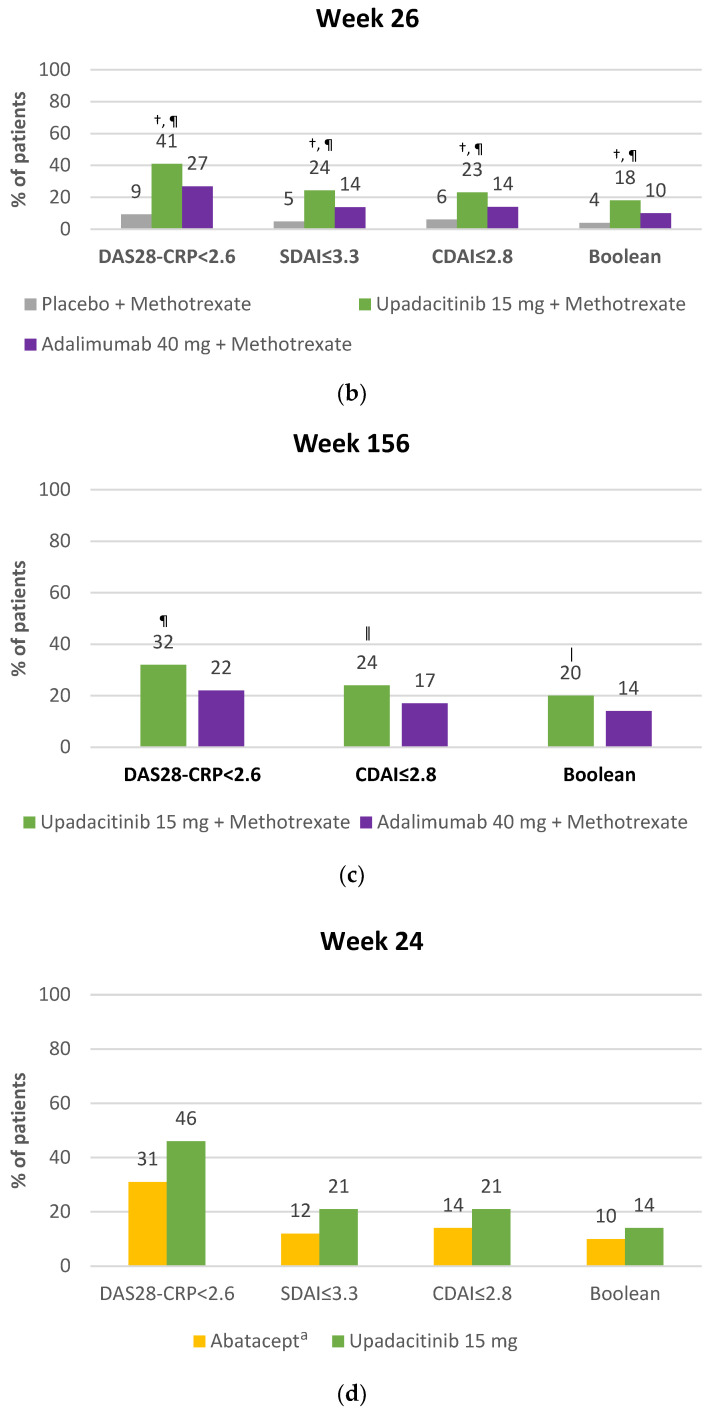
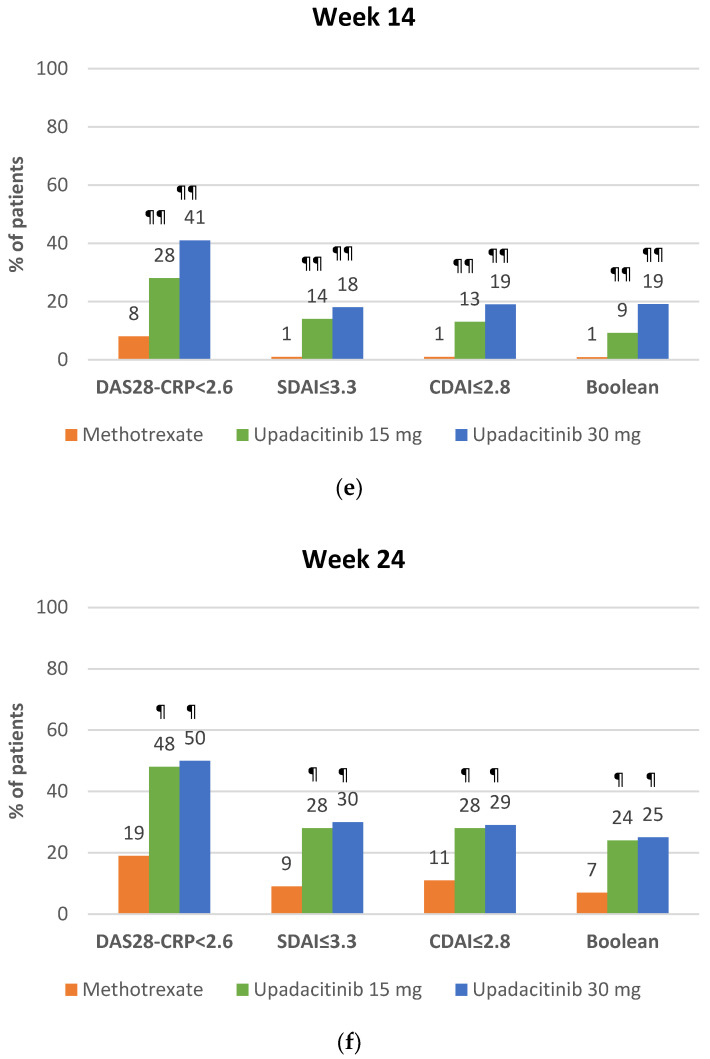
Figure 2.
(a) Clinical remission with upadacitinib in the SELECT-NEXT trial [21]. * p < 0.05 for upadacitinib vs. placebo. ** p < 0.01 for upadacitinib vs. placebo. † p < 0.001 for upadacitinib vs. placebo. ‡ p < 0.0001 for upadacitinib vs. placebo. (b) Clinical remission with upadacitinib in the SELECT-COMPARE trial at week 26 [23]. † p < 0.001 upadacitinib vs. placebo. ¶ p < 0.001 upadacitinib vs. adalimumab. (c) Clinical remission with upadacitinib in the SELECT-COMPARE trial at week 156 [36]. ǀ p < 0.05 upadacitinib versus adalimumab. ǁ p < 0.01 upadacitinib versus adalimumab. ¶ p < 0.001 upadacitinib versus adalimumab. (d) Clinical remission with upadacitinib in the SELECT-CHOICE trial [24]. a No statistical testing was performed. (e) Clinical remission with upadacitinib in the SELECT-MONOTHERAPY trial [25]. ¶¶ p < 0.0001 for upadacitinib versus methotrexate. (f) Clinical remission with upadacitinib in the SELECT-EARLY trial [26]. ¶ p < 0.001 for upadacitinib versus methotrexate. CDAI—clinical disease activity index; DAS28-CRP—disease activity score in 28 joints using C-reactive protein level; SDAI—simplified disease activity index.