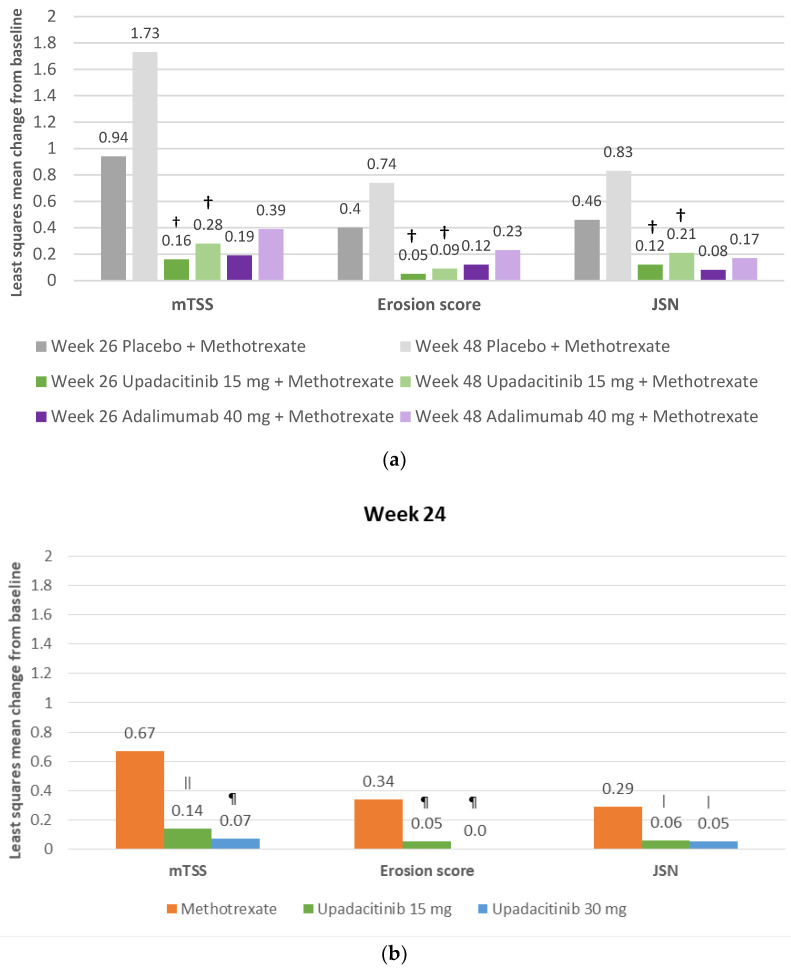
Figure 3.
(a). Radiographic progression in the SELECT-COMPARE trial at week 26 and 48. † 0.001 for upadacitinib versus placebo + methotrexate for the comparisons at each timepoint. Adapted and reproduced from Fleischmann RM, Genovese MC, Enejosa JV, Mysler E, Bes-sette L, Peterfy C, Durez P, Ostor A, Li Y, Song IH. Safety and effectiveness of upadacitinib or adalimumab plus methotrex-ate in patients with rheumatoid arthritis over 48 weeks with switch to alternate therapy in patients with insuffi-cient response. Ann Rheum Dis. 2019 Nov;78(11):1454-1462, © 2019, with permission from BMJ Publishing Group Ltd. (b). Radiographic progression in the SELECT-EARLY trial. ǀ 0.05 upadacitinib versus methotrexate. ǁ 0.01 upadacitinib versus methotrexate. ¶ 0.001 upadacitinib versus methotrexate. Reproduced with permission from van Vollenhoven R, Takeuchi T, Pangan AL, Friedman A, Mohamed MF, Chen S, Rischmueller M, Blanco R, Xavier RM, Strand V. Efficacy and Safety of Upadacitinib Monotherapy in Methotrex-ate-Naive Patients With Moderately-to-Severely Active Rheumatoid Arthritis (SELECT-EARLY): A Multicenter, Multi-Country, Randomized, Double-Blind, Active Comparator-Controlled Trial. Arthritis Rheumatol. 2020 Oct;72(10):1607-1620, © 2020 The Authors. Arthritis & Rheumatology published by Wiley Periodicals LLC on behalf of American College of Rheumatology. JSN—joint space narrowing; mTTS—modified total Sharp/van der Heijde score.