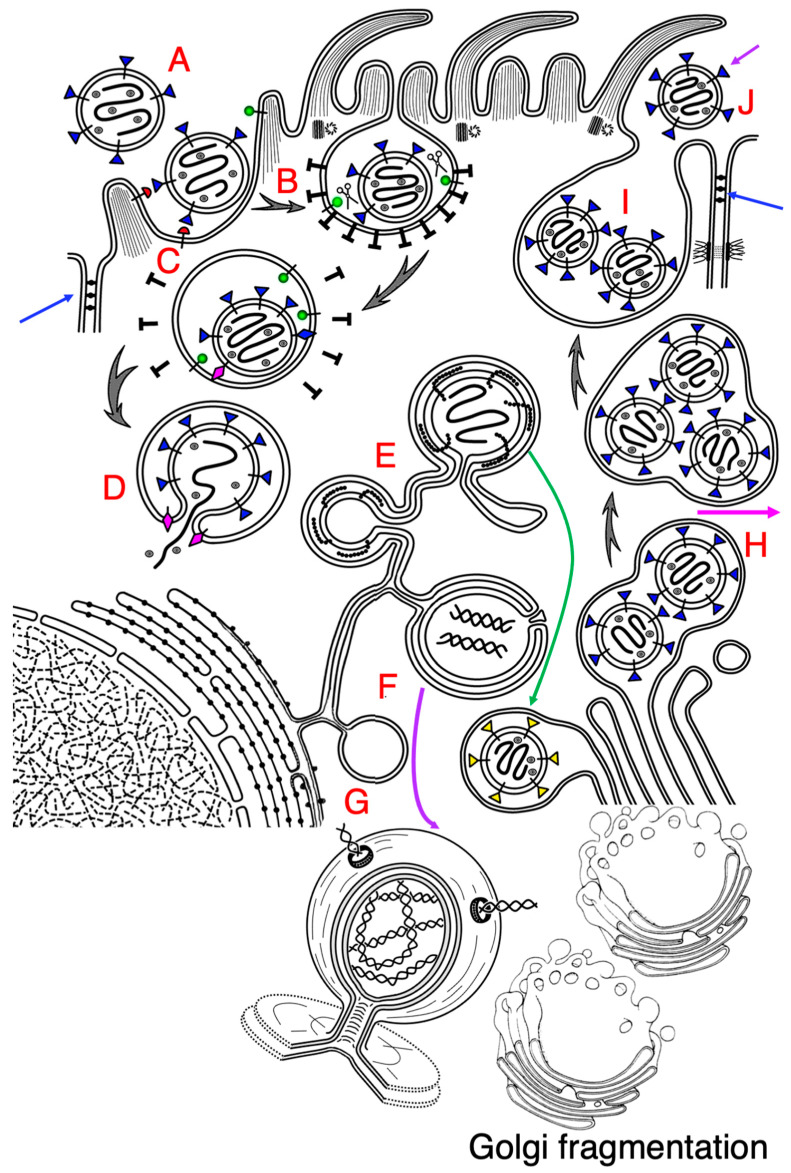
Figure 1.
Biogenesis of SARS-CoV-2. The scheme shows interactions of SARS-CoV-2 with an epithelial ciliated cell. (A) After attachment of the virion to ACE2 (transmembrane structure with a hemispherical red head). (B) Clathrin-dependent endocytosis indices invagination of the apical plasma membrane and formation of endosome (C). Then, S-protein of SARS-CoV-2 (transmembrane structures with the triangle blue heads) is subjected to cleavage with TMDRSS2 (transmembrane structure with a spherical green head). It (magenta rhomb) perforates the endosomal limiting membranes (D). RNA of SARS-CoV-2 enters the cytosol (D) and forms the zippered endoplasmic reticulum (E). Simultaneously, double membrane vacuoles (DMV; (F,G)) are formed. (G) DMVs are filled with dsRNA and contain pores, through which dsRNA can move. Budding of the virus occurs within the ER exit site and ZER (E). During budding, the S-protein appears to be not fully glycosylated. After their budding, the viral particles are delivered to the Golgi complex and transported through it (green arrow). The immature (without glycosylation) virus passes through the GC, where it is subjected to high level of glycosylation. Viral particles are enriched (their numerical density increases) in the post-Golgi compartment (H). After concentration of viruses at the trans-side of the Golgi complex, the post-Golgi vacuole is delivered to the apical plasma membrane and fuses with it (I), and it secretes viruses into the lumen of airway ((J); magenta arrow) or into the space between epithelial cells (violet arrow). Blue arrows indicate tight junctions. SARS-CoV-2 induces fragmentation of the Golgi complex (see below to the right).