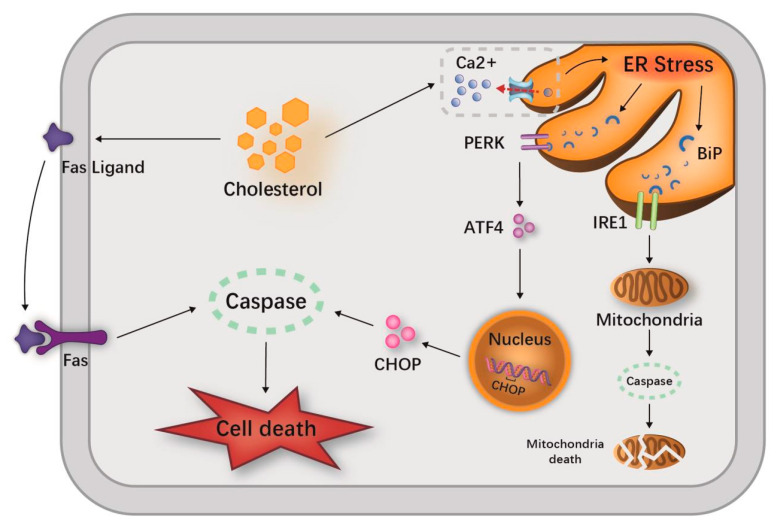
Figure 4.
Several pathways of cholesterol-induced cell death. The abnormal loading of cholesterol in the ER membrane leads to the exhaustion of ER calcium stores and ER stress, initiating the UPR and ultimately apoptosis, as well as the release of the ER chaperone BiP, which activates the UPR sensors PERK and IRE1. Activated PERK mediates the translation of ATF4 and promotes the expression of CHOP. Activated IRE1 induces a mitochondria-dependent caspase cascade. ER stress also promotes caspase-12 cleavage and activation, initiating caspase activation and leading to programmed cell death. Free cholesterol can also trigger apoptosis though the Fas pathway. Abbreviations: IRE1, inositol requiring enzyme 1; BiP, binding immunoglobulin protein; PERK, protein kinase (PKR)-like ER kinase; ER, endoplasmic reticulum; ATF4, activating transcription factor 4; CHOP, C/EBP homologous protein; ER, endoplasmic reticulum; UPR, unfolded protein responses.