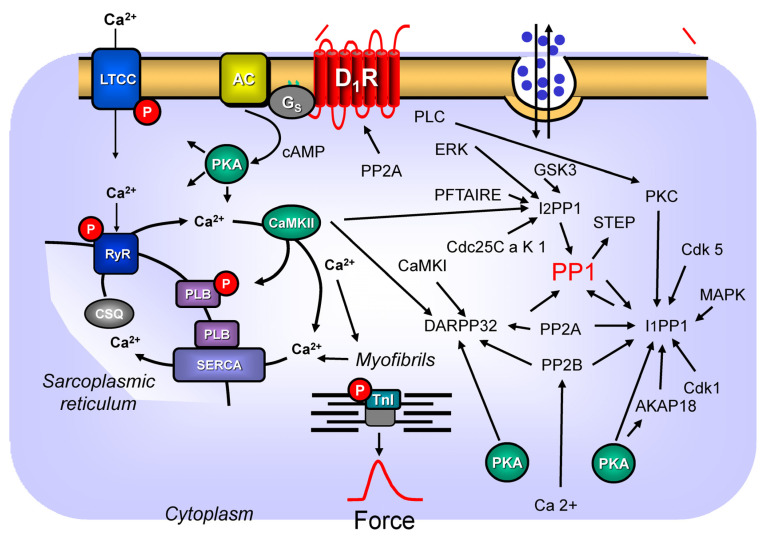
Figure 2.
Speculative mechanisms of D1-dopamine receptor-mediated signal transduction in the heart. These pathways are known in the central nervous system, but they need to be elucidated in the human heart. Protein phosphorylations can be reversed via the action of protein phosphatases, such as PP1, PP2A, or PP2B. The so-called “dopamine- and cAMP-regulated phosphoproteins with an apparent weight of 32 kDa” (DARPP32), protein phosphatase-1-inhibitor-1 (I-1) protein phosphatase-1-inhibitor-2 (I-2), can inhibit PP1 and thus amplify the function of the D1-dopamine receptor. The inhibitory actions of I-1 and DARPP32 on PP1 are amplified after their phosphorylation by cAMP-dependent protein kinase (PKA). I-1 and DAPRP32 are dephosphorylated, and thus they are inactivated by PP2A and PP2B. PP2A might increase the insertion of D1-dopamine receptors (D1-R) into the sarcolemma. I-2 can be regulated in its activity to inhibit PP1 by the kinases shown here: casein kinase II (CamKII), glycogen synthase kinase 3 (GSK-3), a kinase abbreviated as PFTAIRE kinase (PFTAIRE), by ERK, and a Cdc25C-associated kinase 1 (Cdc25C a K). I-1 is phosphorylated, and its activity is altered by Cdk1, CdK5, protein kinase C (PKC), and a mitogen-activated kinase (MAPK). See text for further details.