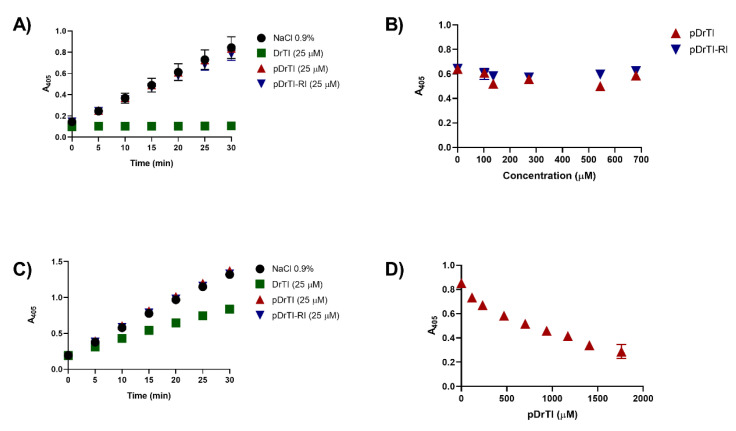
Figure 1.
Inhibitory activity—Bovine trypsin (37 nM) activity was performed with the synthetic substrate BAPNA (Bz-Arg-pNA). Substrate hydrolysis was measured through p-nitroaniline release by reading on a plate spectrophotometer at a wavelength of 405 nm every 5 min (A) or the end of the 30 min (B) with different concentrations of the peptides under study. PKa (14.7 nM) activity was performed with the synthetic substrate HD-Pro-Phe-Arg-pNa. Substrate hydrolysis was measured through p-nitroaniline release by reading on a plate spectrophotometer at a wavelength of 405 nm every 5 min (C) or the end of the 30 min (D) with different concentrations of the peptides under study. Assays were done in triplicate.