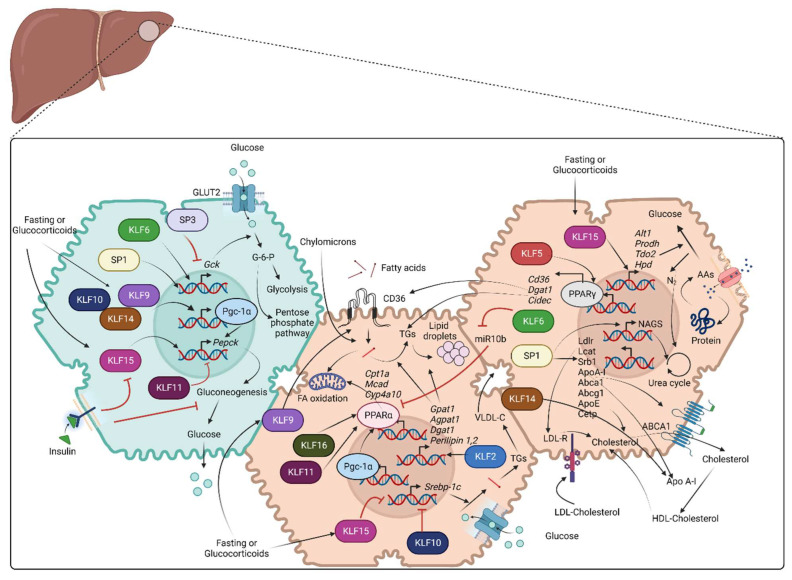
Figure 2.
Role of SP/KLF transcription factors in metabolism: Green shade represents glucose metabolism, and brown shade represents lipid and amino acid metabolism. Glucose metabolism: Glucose enters hepatocytes through GLUT2 and is converted to glucose-6-phosphate with the contribution of the Gck enzyme. Glucose-6-phosphate further participates in glycolysis or pentose phosphate pathway. SP1 and KLF6 stimulate the transcription of Gck. Fasting or glucocorticoid stimulation induces KLF9, KLF10, KLF14, and KLF15. KLF9, KLF10, and KLF14 stimulate transcription of Ppargc1a, whereas KLF15 along with PGC-1α, promotes the transcription of Pepck that encodes the rate-limiting step of gluconeogenesis, leading to increased hepatic glucose production. KLF11 suppresses gluconeogenesis via transcriptional repression of Pepck and Ppargc1a. Lipid metabolism: Fatty acid uptake is mediated by CD36 and is followed by TG storage in lipid droplets or β-oxidation in mitochondria. Hepatocytes carry out de novo lipogenesis from pyruvate and the synthesis of cholesterol that is secreted as a component of VLDL or HDL particles. SP1 stimulates the transcription of several genes that are important for cholesterol metabolism, including LDLR, LCAT, SRB1, APOA-I, ABCA1, ABCG1, APOE, and CETP. KLF14 stimulates the transcription of APOA-I. KLF2 and KLF9 promote lipid uptake via transcription of Cd36. KLF2 promotes the transcription of enzymes of TG synthesis and lipid storage (Gpat1, Agpat1, Dgat1, Perilipin 1, Perilipin 2). KLF11 and KLF16 promote β-oxidation of fatty acids by facilitating PPARα-mediated transcription of Cpt1a, Mcad, Cyp4a10, and Cyp4a14. KLF6 indirectly stimulates PPARα-mediated transcription of Trb3 by suppressing transcription of miR10b. KLF10 and KLF15 suppress SREBP-1c-mediated hepatic lipogenesis. KLF5 promotes the transcription of genes for lipid uptake and metabolism (Cd36, Dgat1, Cidec) through PPARγ stimulation. Amino acid/protein metabolism: Hepatocytes obtain circulating AAs through transporters or from degraded protein/polypeptides and use their carbon backbone for gluconeogenesis. Ammonia released from this reaction is toxic and enters the urea cycle. SP1 enhances the transcriptional induction of NAGS, which is essential for the urea cycle. On the other hand, KLF15 encodes transcription of the enzymes Alt1, Prodh, Tdo2, and Hpd, which are essential for AA metabolism and, eventually, gluconeogenesis (AA: Amino acids, Abca1: ATP binding cassette subfamily A member 1, Abcg1: ATP binding cassette subfamily G member 1, Agpat1: 1-acyl-sn-glycerol-3-phosphate acyltransferase alpha, Alt1: Alanine aminotransaminase 1, Apo A-I: Apolipoprotein A-I, ApoE: Apolipoprotein E, Cetp: Cholesteryl ester transfer protein, Cidec: Cell death-inducing DFFA-like effector C, Cpt1a: Carnitine palmitoyltransferase 1A, Cyp4a10: Murine cytochrome P450, family 4, subfamily a, polypeptide 10, Dgat1: Diacylglycerol O-Acyltransferase 1, FA: Fatty acids, Gck: Glucokinase, GLUT2: Glucose transporter 2, Gpat1: Glycerol-3-phosphate acyltransferase 1, HDL: High density lipoprotein, Hpd: 4-Hydroxyphenylpyruvate dioxygenase, KLFs: Krupple like family transcription factors, Lcat: Lecithin-cholesterol acyltransferase, Ldlr: Low-density lipoprotein receptor, Mcad: Medium-chain acyl-CoA dehydrogenase, NAGS: N-acetyl glutamate synthase, PGC-1α: Peroxisome proliferator-activated receptor-gamma coactivator-1alpha, PPARα: Peroxisome proliferator-activated receptor alpha, PPARγ: Peroxisome proliferator- activated receptor gamma, Prodh: Proline dehydrogenase, SP: Specificity protein family transcription factors, Srb1: Scavenger receptor class B type 1, Srebp-1c: Sterol regulatory element-binding protein 1, Tdo2: Tryptophan 2,3-dioxygenase, TG: Triglycerides, VLDL: Very low density lipoprotein).