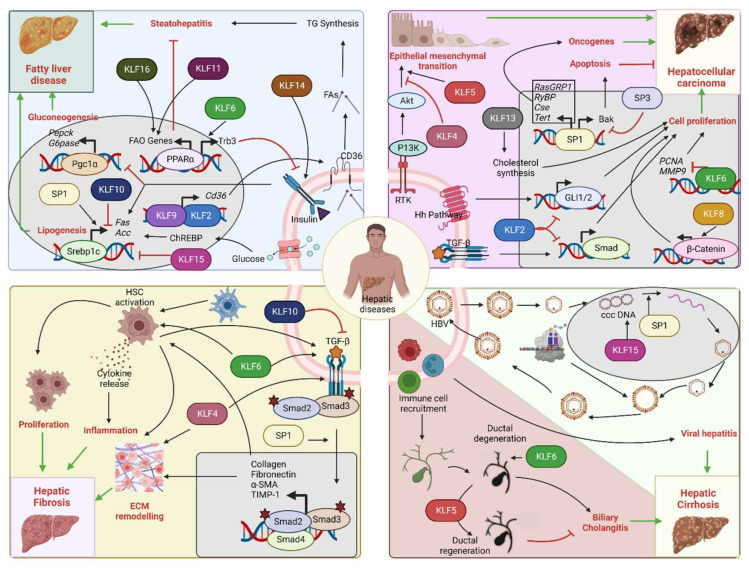
Figure 4.
Role of SP/KLF transcription factors in hepatic diseases: Upper left corner: Insulin resistance/NAFLD—Insulin signaling promotes SREBF-1-mediated lipogenesis and inhibits PGC-1α-mediated gluconeogenesis. Impaired insulin signaling leads to fatty liver disease. KLF2 and KLF9 drive Cd36 expression that increases fatty acid uptake and steatohepatitis. KLF11 and KLF16 drive PPARα-mediated fatty acid oxidation gene expression and prevent steatohepatitis. KLF6 interferes with insulin signaling through PPARα-mediated Trb3 expression. KLF14 promotes insulin signaling through PI3K-Akt. SP1 increases lipogenesis, while KLF10 and KLF15 inhibit it. Upper right corner: HCC—SP1 promotes transcription of oncogenes, such as RasGRP1, RYBP, CSE, and TERT, and facilitates HCC progression. SP1 can also increase the expression of the apoptotic gene Bak, hence promoting tumor suppression. SP3 opposes SP1 action on Bak expression. KLF2 is a tumor suppressor by inhibiting Hedgehog and TGF-β-Smad pathways. KLF8 and KLF13 promote tumor proliferation by activation of Wnt/β-catenin and cholesterol synthesis. KLF6 also acts as a tumor suppressor by inhibiting PCNA and MMP9 oncogene transcription. KLF5 facilitates receptor tyrosine kinase-mediated EMT, whereas KLF4 opposes it. Bottom left corner: Hepatic fibrosis—HSC activation promotes hepatic fibrosis by altering the ECM composition and releasing inflammatory cytokines. SP1 promotes fibrosis through stimulation of the TGF-β pathway. KLF4 and KLF6 promote fibrosis by facilitating the HSC actions on ECM remodeling and the TGF-β pathway. KLF10 opposes the actions of the TGF-β pathway and hence prevents the progression of fibrosis. Bottom right corner: Viral hepatitis and biliary cholangitis: HBV infection, ccc DNA replication, and coat protein synthesis lead to the formation of virion particles that are finally released. SP1 facilitates the replication of ccc DNA. KLF15 activates the promoter of surface proteins and core genes. Immune cell-mediated damage of biliary epithelial cells causes bile duct degeneration and cholangitis. KLF6 facilitates the development of cholangitis through excess bile acid secretion and accumulation, while KLF5 prevents it by promoting biliary epithelial cell remodeling and ductal regeneration (Acc: Acetyl coA carboxylase, Akt: Akt serine/threonine kinase, Bak: Bcl-2 homologous antagonist/killer, ccc DNA: Covalently closed circular DNA, CD36: Fatty acid translocase, ChREBP: Carbohydrate response element binding protein, Cse: Cystathionine γ-lyase, ECM: extracellular matrix, FA: Fatty acids, FAO: Fatty acid oxidation, Fas: Fatty acid synthase, GLI1/2: Glioma-associated oncogene 1/2, G6Pase: Glucose 6 phosphatase, HBV: Hepatitis B virus, Hh: Hedgehog, HSC: Hepatic stellate cell, KLFs: Krupple like family transcription factors, MMP9: Matrix metalloproteinase 9, PCNA: Proliferating cell nuclear antigen, Pepck: Phosphoenol pyruvate carboxykinase, Pgc-1α: Peroxisome proliferator-activated receptor-gamma coactivator-1alpha, PI3K: Phosphoinositide 3-kinases, PPARα: Peroxisome proliferator-activated receptor alpha, RasGRP1: RAS guanyl-releasing protein 1, Rtk: Receptor tyrosine kinase, RYBP: RING1 And YY1 binding protein, Smad: Suppressor of mothers against decapentaplegic, SP: Specificity protein family transcription factors, Tert: Telomerase reverse transcriptase, TG: Triglycerides, TGF-β: Transforming growth factorβ, Trb3: Tribbles pseudokinase 3).