Figure 4: cBAF action requires AQ and IDR domains of PU.1.
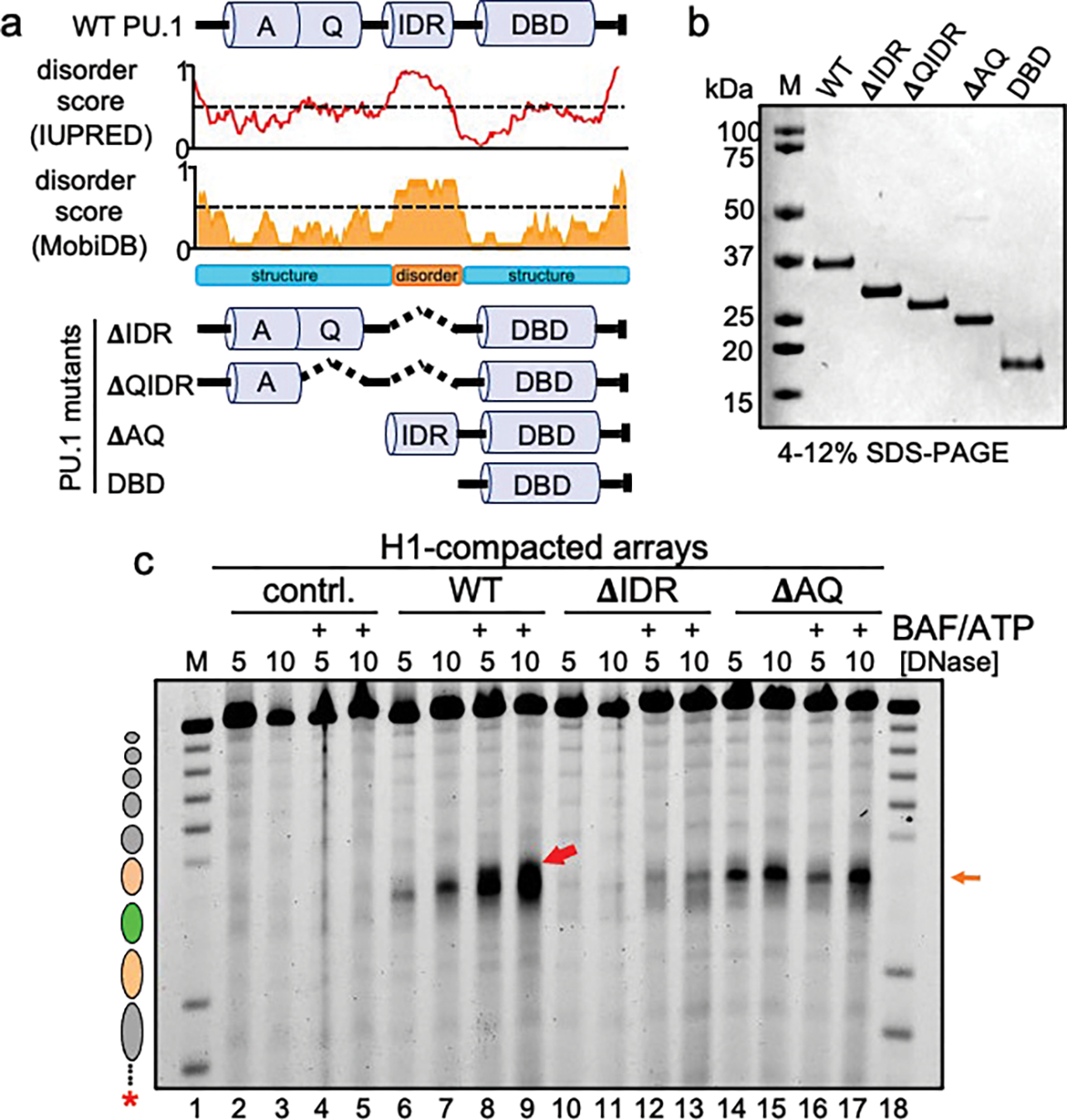
a, Predicted disorder tendency of WT PU.1. The red and orange lines indicate the predicted disorder tendency along the WT PU.1 protein (as calculated by IUPred and MobiDB, respectively) with values above 0.5 (indicated by the dotted line) considered disordered. Shown are the positions of the Acidic domain (A), Q-rich domain (Q), Intrinsically disordered region (IDR) and DNA-binding domain (DBD). The deletion mutant series of PU.1 are indicated below as ΔIDR, ΔQIDR, ΔAQ, and DBD. b, SDS-PAGE analysis of 1 μg of each protein, stained with Coomassie blue. c, DNase I digestion analysis H1-compacted nucleosome arrays incubated alone (contrl., lanes 2–5), WT PU.1 (lanes 6–9), ΔIDR deletion (lanes 10–13), and ΔAQ truncation (lanes 14–17) with or without the addition of BAF and ATP.
