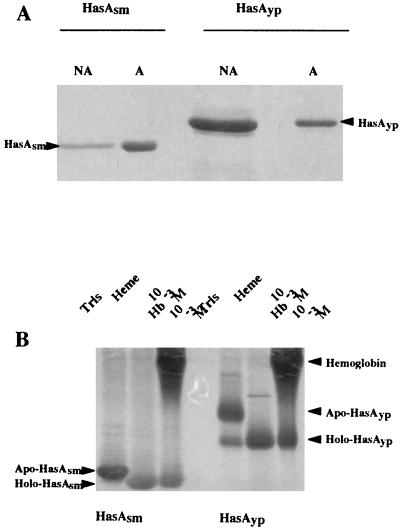
FIG. 4.
HasAyp is a heme-binding protein. (A) Isolation of HasAyp by heme affinity chromatography. Coomassie blue staining is shown. Concentrated supernatants were incubated with hemin-agarose as described in Materials and Methods. (Left) HasAsm, NA, E. coli MC4100(pSyc150, pHasAsm) concentrated supernatant not adsorbed (flowthrough material in excess) on hemin-agarose; A, E. coli MC4100(pSyc150, pHasAsm) concentrated supernatant adsorbed on hemin-agarose. (Right) HasAyp, NA, E. coli MC4100(pSyc150, pHasAyp) concentrated supernatant not adsorbed on hemin-agarose; A, E. coli MC4100(pSyc150, pHasAyp) concentrated supernatant adsorbed on hemin-agarose. The presence of HasAsm and HasAyp in NA lanes indicates that the capacity of the hemin-agarose columns was saturated by the supernatant concentrates used here. (B) Heme acquisition and formation of holoHasAsm and holoHasAyp after incubation of HasAsm or HasAyp with heme or hemoglobin. Four microliters (1 μg/ml) of HasAsm prepared from ammonium sulfate-concentrated supernatants of E. coli MC4100(pSyc150, pHasAsm) and 2 μl of HasAyp (2 μg/ml) prepared from ammonium sulfate-concentrated supernatant of E. coli MC4100(pSyc150, pHasAyp) were incubated with equal volumes of heme or hemoglobin (10−3 M) for 45 min at room temperature. The mixtures were loaded onto a 15% polyacrylamide gel and electrophoresed in the absence of SDS. The gel was stained with Coomassie blue. Protein bands complexed with heme (holoproteins) have a faster migration rate than uncomplexed protein (apoproteins).