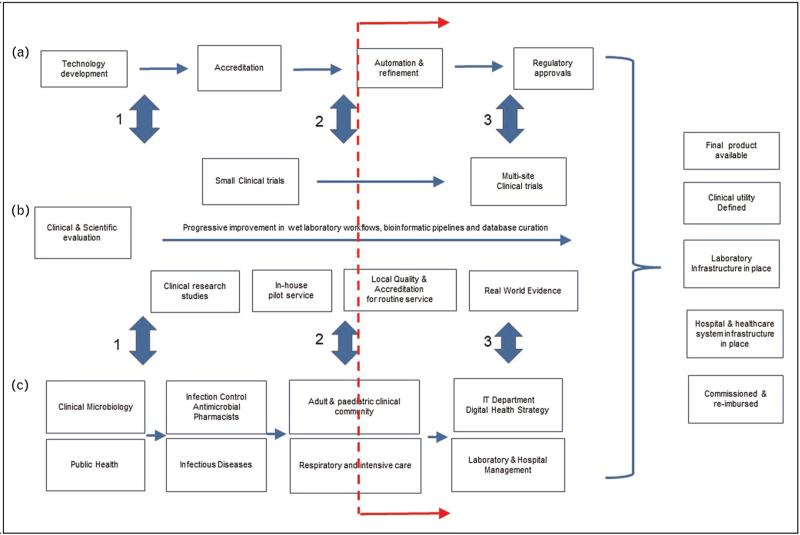
FIGURE 1.
Integrating contribution from different stakeholders to the respiratory metagenomics route to routine service. This is an illustrative perspective from a clinical academic laboratory. Stakeholders will use different terminologies and have different views on ordering and prioritisation of activities which are not necessarily sequential. The core principle is close iterative engagement between each workstream. (a) Industry workstream involves technology development, ISO9001 or ISO13485 accreditation of their products and submission to regulators. (b) Basic, translational and clinical scientist workstream. Involves technology co-development, workflow refinement to optimise performance characteristics, evaluation in small laboratory studies using clinical samples, pilot local service evaluation of internally developed workflows under clinical governance framework, through to ISO15189 accreditation as a laboratory developed test generating real world evidence and involvement in large clinical trials (c) Clinical and service user workstream of all hospital based stakeholders including service providers (microbiology and infectious diseases), infection specialty service users (infection control & antimicrobial stewardship), clinical users (respiratory and intensive care physicians), service enables (IT) and internal decision makers and funders (hospital management). Three main stages of close inter-workstream engagement are represented by vertical filled arrows as (1) Proof-of-concept stage (circa 50–100 sample studies) that a workflow can be safely and reliably performed delivering results that have potential for clinically utility in a required timeframe. This gives support for each workstream to progress to (2) Proof-of-value (circa 500–1000 sample studies) involving early clinical evaluation generating real-world evidence and assessment as a locally developed and accredited test with defined performance characteristics, which informs 3) Proof-of-implementation requiring investment from industry in final product development and regulatory approval, by research funders to support large multisite clinical trials to demonstrate clinical utility and cost-effectiveness and, by healthcare systems implementing infrastructure, producing business cases for using tests, training staff and re-organising clinical pathways to realise benefits. Dotted red line indicates approximate current position.