Abstract
The existence of neuroinflammation and oxidative stress surrounding amyloid beta (Aβ) plaques, a hallmark of Alzheimer’s disease (AD), has been demonstrated and may result in the activation of neuronal death and inhibition of neurogenesis. Therefore, dysregulation of neuroinflammation and oxidative stress is one possible therapeutic target for AD. Kaempferia parviflora Wall. ex Baker (KP), a member of the Zingiberaceae family, possesses health-promoting benefits including anti-oxidative stress and anti-inflammation in vitro and in vivo with a high level of safety; however, the role of KP in suppressing Aβ-mediated neuroinflammation and neuronal differentiation has not yet been investigated. The neuroprotective effects of KP extract against Aβ42 have been examined in both monoculture and co-culture systems of mouse neuroectodermal (NE-4C) stem cells and BV-2 microglia cells. Our results showed that fractions of KP extract containing 5,7-dimethoxyflavone, 5,7,4′-trimethoxyflavone, and 3,5,7,3′,4′-pentamethoxyflavone protected neural stem cells (both undifferentiated and differentiated) and microglia activation from Aβ42-induced neuroinflammation and oxidative stress in both monoculture and co-culture system of microglia and neuronal stem cells. Interestingly, KP extracts also prevented Aβ42-suppressed neurogenesis, possibly due to the contained methoxyflavone derivatives. Our data indicated the promising role of KP in treating AD through the suppression of neuroinflammation and oxidative stress induced by Aβ peptides.
Keywords: Alzheimer’s disease, Kaempferia parviflora, microglia, neuronal differentiation, neuroinflammation
1. Introduction
Dementia is a type of neurodegenerative disease comprising frontotemporal dementia, Lewy body dementia, vascular dementia, and Alzheimer’s disease (AD), with AD accounting for 60–70% of all dementia cases [1]. Dementia, including AD, is characterized by neuronal loss, leading to a decline in memory and cognitive ability that negatively impact individuals and their families [1]. AD etiology is multifaceted, with several variables including genetic factors, reduced neurogenesis, mitochondrial dysfunction, oxidative stress accumulation, neuroinflammation, and accumulation of hyperphosphorylated tau and amyloid beta (Aβ) peptides. These variables interact and generate a vicious cycle that accelerates neuronal damage and death, leading to neuron loss and brain atrophy [2,3]. The neuroinflammatory responses surrounding Aβ plaques in AD suggest that inflammation and sustained immune responses activated by microglia and reactive astrocytes are disease characteristics [2,4].
Neural stem cells can multiply and differentiate into many cell types in the nervous system such as oligodendrocytes, microglia, and neurons. Several studies showed that Aβ peptides reduce neural stem cell survival and proliferation and decrease neurogenic differentiation [5,6,7]; thus, neurogenesis dysfunction could be considered a hallmark of AD [2,8]. Currently, the US Food and Drug Administration (FDA) has approved AD medicines including donepezil, galantamine, rivastigmine, and memantine. The first three act as cholinesterase inhibitors, while memantine acts as a blocking agent for N-methyl-D-aspartate (NMDA) receptors. However, these medicines cannot treat behavioral and psychiatric symptoms in moderate and severe stages of AD [9], and suppression of Aβ-mediated neuroinflammation with improved survival of neural stem cells is an alternative AD therapy. Ginkgo biloba extract, curcumin, garcinol, gallic acid, and ginsenosides have all been reported for their anti-AD properties through the reduction of Aβ-mediated neuroinflammation and the ability to modify the process of neural differentiation [10,11,12,13].
Kaempferia parviflora Wall. ex Baker (K. parviflora; KP) is a perennial plant belonging to the Zingiberaceae family, like turmeric (Curcuma longa L.) and ginger (Zingiber officinale Roscoe). The KP rhizome is mostly employed as a health-promoting substance in traditional medicine to treat a broad range of illnesses including anti-osteoarthritis, anticancer, anti-psoriasis, anti-obesity, antioxidative stress, anti-AD, and anti-inflammation [14,15,16,17,18]. An ethanolic extract of KP reduced gene and protein expression of inflammatory markers including interleukin-1β (IL-1β), interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α) in lipopolysaccharide (LPS)-stimulated macrophages through inhibition of the nuclear factor-κB (NF-κB) translocation [14], while 5,7,4′-trimethoxyflavone (TMF) and 5,7-dimethoxyflavone (DMF) isolated from KP exhibited potential enzyme inhibitory activities against acetylcholinesterase (AChE) and butyrylcholinesterase (BChE), which are drug targets for AD treatment as mentioned above [19]. Moreover, the ethanolic extract of KP containing 3,5,7,3′,4′-pentamethoxflavone (PMF), 5,7,4′-trimethoxyflavone, and 5,7-dimethoxyflavone alleviated cognitive deficits and prevented a decrease in neural progenitor cell division caused by valproic acid (VPA) administration in rats [20]. VPA is an antiepileptic medication that inhibits cell proliferation and neurogenesis in the hippocampus [21] that causes moderate to severe cognitive impairments [22]. KP has also been examined for its toxicity and effectiveness. KP extract had a no-observed-adverse-effect-level (NOAEL) of >249 mg/kg BW/day according to sub-chronic testing and was also free of genotoxicity, suggesting a high degree of safety [23]. Overall, KP extract showed promise as a natural, safe ingredient to treat anti-inflammatory and anti-AD activities by inhibiting AChE, BChE, and neurogenesis decline. Therefore, this study hypothesized that KP extract may play a role in suppressing Aβ-mediated neuroinflammation and neuronal differentiation as a novel mechanism for the prevention and/or reduction of inflammation-induced AD. This is the first investigation to determine which KP fractions can counteract Aβ effects through the activity of neural stem cells, microglial cells, and differentiated neurons. Data from single cell culture and co-culture between neuronal stem cells (NE-4C) and microglial cells (BV-2) were also compared to observe the relationship between them that partially mimicked neuroglia. Results showed that KP exerted anti-neuroinflammatory properties with neuroprotective potential and promoted neuronal differentiation against Aβ.
2. Materials and Methods
2.1. Chemicals
Cell culture medium and supplements, such as minimum essential medium (MEM), Roswell Park Memorial Institute medium 1640 (RPMI-1640), neurobasal medium, glutamine, penicillin, streptomycin, fungizone, and trypsin, were acquired from Invitrogen (Waltham, MA, USA). B-27 and fetal bovine serum (FBS) were purchased from Thermo Fisher Scientific (Waltham, MA, USA) and HyClone (Logan, UT, USA), respectively. The IL-6 ELISA kit was purchased from Abcam (Cambridge, MA, USA). The Griess reagent and CellTiter-Glo® Luminescence assay kit were purchased from Promega (Madison, WI, USA). Tri-RNA Reagent was purchased from Favorgen (Kaohsiung, Taiwan). The 2× qPCRBIO SyGreen 1step Lo-ROX was obtained from PCR Biosystems (Wayne, PA, USA). 2,3-Bis-(2-Methoxy-4-Nitro-5-Sulfophenyl)-2HTetrazolium-5-Carboxanilide (XTT), phenazine methosulfate (PMS), Aβ42 peptides, hexafluoropropanol, poly-l-lysine (PLL), dichlorofluorescein diacetate (DCFDA), were obtained from Sigma-Aldrich (St. Louis, MO, USA). Unless otherwise noted, all additional reagents and standards were from Sigma-Aldrich.
2.2. Preparation of Amyloid Beta Peptides (Aβ42)
Aβ42 was prepared using previous procedures with some modifications [24,25,26]. In brief, Aβ42 peptides were dissolved in hexafluoropropanol. Subsequently, the solution was aliquoted, dried in a speed-vacuum centrifuge, and stored at −80 °C. The dried peptide was reconstituted in 5 mM dimethyl sulfoxide (DMSO) and diluted with phosphate-buffered saline (PBS) to reach a concentration of 100 μM. The solution was then allowed to aggregate for 72 h at 37 °C and subsequently aliquoted and kept at −80 °C until use.
2.3. Plant Extraction and Characterization
Kaempferia parviflora Wall. Ex Baker (K. parviflora; KP) was purchased from a traditional Thai pharmacy in Bangkok, Thailand, in 2018. For preparing the extract, dried KP was pulverized into coarse powder and soaked with 95% ethanol for 96 h [27,28]. The filtrate was evaporated and freeze-dried to obtain the crude ethanolic KP extract (KP1). The crude extracted was dissolved in 50% ETOH, then partitioned with hexane, chloroform, and ethyl acetate, and then evaporated and freeze-dried, obtaining hexane (KP2), chloroform (KP3), ethyl acetate (KP4), and residue (KP5) fractions. The extracts were kept at −80 °C until used.
Phenolic derivatives and 5,7-dimethylflavone (DMF) contents in the extract were determined by high-performance liquid chromatography (HPLC) using a C18 column (250 mm × 4.6 mm, 5 µm) (Agilent Technologies, Santa Clara, CA, USA). Gradient elution for detection was performed using two solvents, A (1% acetic acid in water) and B (100% acetonitrile). Twenty microliters of 10 mg of KP extract and its fractions were dissolved in 1 mL of diH2O, injected into the column with a flow rate of 0.7 mL/min, and detected at 280 nm. The peak area and retention time of the sample were determined and compared with authentic standards including catechin, caffeic acid, rutin, rosmarinic acid, quercetin, apigenin, and kaempferol.
High-performance liquid chromatography (HPLC) was performed using the above column to determine the existence of flavone derivatives, DMF (5,7-dimethoxyflavone), PMF (3,5,7,3′,4’-pentamethoxyflavone), and TMF (5,7,4’-trimethoxyflavone) contents in the KP crude extract and its fractions. The flavone derivatives were separated using a gradient system of mobile phase A (water) and mobile phase B (100% methanol) with a total run time of 60 min and flow rate of 0.6 mL/min. The gradient system was 35% A in 0–35 min, followed by 20% A in 40–55 min and 35% A in the next 60 min. Then, 10 mg/mL of the extract dissolved in 1 mL of methanol was injected into the column and detected at 254 nm. Peak areas and retention times of the samples were evaluated and compared with the standard curve of the authentic standards (PMF, DMF, and TMF).
2.4. Cell Cultures
2.4.1. Monoculture of Neural Stem Cells (NE-4C)
Mouse neuroectodermal (NE-4C) stem cell line (CRL-2925) was obtained and cultured according to ATCC culture method guidelines (Manassas, VA, USA). Briefly, cells were maintained in MEM supplemented with 5% FBS 2 mM glutamine, 100 units/mL penicillin, and 100 µg/mL streptomycin at 37 °C with 5% CO2. Cells were plated on a 0.01% PLL-coated culture flask at a cell density of 104 cells/cm2 and subcultured at a 1:10 ratio. Supplemented MEM was changed every two days.
2.4.2. Monoculture of Microglia Cells (BV-2)
BV2 cells, a mouse microglial cell line, were purchased from Interlab Cell Line Collection (ATL033001, Genova, Italy). The cells were cultured and seeded according to the previous reports [29,30].
2.4.3. Differentiation of NE-4C Neural Stem Cells into Neurons
The differentiation of NE-4C stem cells into neurons was performed according to the previous protocol [31]. Briefly, NE-4C cells were dissociated with 0.05% trypsin-EDTA and plated onto PLL-precoated 24-well plates at 1.5 × 106 cells/well. The cells were cultured in supplemented MEM for 12 h before neuronal differentiation induction. Differentiation was induced by replacing the MEM with a neurobasal medium supplemented with 100 units/mL penicillin, 100 µg/mL streptomycin, and B-27. The supplemented neurobasal medium was replaced every 48 h. Six days after induction, aggregates with clusters of neural stem cells were mechanically dissociated, seeded onto PLL-coated 24-well plates, and grown until day twelve.
2.4.4. Co-Culture System between NE-4C Stem Cells and BV-2 Microglial Cells
Co-culture of NE-4C stem cells and microglial cells was performed using 24-well insert plates (Millipore, Burlington, MA, USA). NE-4C cells were cultured in PLL-coated 24-well plates at a density of 1.5 × 106 cells/well in supplemented MEM at the bottom of the well, while microglia were cultured in supplemented RPMI at a density of 1 × 105 cells/well in the insert for 24 h.
Induction of neuronal differentiation in the co-culture system between NE-4C stem cells and BV-2 microglial cells was performed by replacing the media with the supplemented neurobasal medium. The BV-2 cells were loaded for co-culture with the plated NE-4C cells and shared media of the supplemented neurobasal medium. After six days, neurospheres were collected and dissociated, seeded onto PLL- coated 24-well plates without co-cultured microglial cells, and grown until day twelve.
2.4.5. Co-Culture System between Differentiated Neurons Derived from NE-4C Cells and BV-2 Microglial Cells
NE-4C stem cells were differentiated into neuronal cells and cultured in supplemented neurobasal medium for 12 days to promote neuronal maturation in the bottom of the well. Consequently, the BV-2 cells in the insert were loaded as an upper chamber for co-culture with the matured neurons and the shared media of neuronal cells.
2.5. Cytotoxic Effect of Amyloid Beta Peptides and KP Extracts
BV-2 and NE-4C cells were seeded in a 96-well plate at 1 × 104 and 2.0 × 104 cells/well, respectively, and incubated overnight using the 12-day-differentiated neuron-derived NE-4C cells at 1.5 × 106 cells/well grown in a 24-well plate. Based on the previous studies, the cells were treated with various concentrations of Aβ42 solution (0.25, 0.5, 1, 5, 10 and 20 µM) [5,26,32,33,34,35]. The cytotoxicity of KP extract (KP1–KP5) ranges from 0.5 ng/mL−1 mg/mL was tested in the cells for 24 h. An equivalent quantity of 0.05% DMSO was used as a vehicle control.
2.6. Protective Effects of KP Extracts in Both Mono and Co-Culture
The protective effects of KP extract on Aβ42-induced cytotoxicity were performed for both monocultures (BV-2 cells, undifferentiated NE-4C cells and differentiated neurons) and co-cultured (BV-2 cells cultured with undifferentiated NE-4C cells, and BV-2 cells cultured with differentiated NE-4C cells) as described. Aβ42 at 5 µM, which reduced 50% of cellular ATP level, was cotreated with KP extracts at nontoxic concentrations (0.5, 1, 2, 4, and 8 µg/mL) and incubated for 24 h. After exposure, the XTT reduction assay and ATP level were utilized to evaluate cytotoxicity.
2.7. Anti-Inflammatory Activities of KP Extracts on Aβ42-Induced Inflammation in Monoculture
The BV-2 cells were seed in a 96-well plate at 1 × 104 cells/well and co-incubated with 1 µM Aβ42 with or without KP extract at nontoxic concentrations (0.5, 1, 2, 4 and 8 µg/mL) for 24 h. BV-2 cells were harvested to determine the intracellular reactive oxygen species (ROS) using the DCF fluorescence assay, while the culture supernatant was collected and determined for IL-6 and nitrite levels.
2.8. Anti-Inflammatory Effects of KP Extracts on Aβ42-Induced Inflammation in Co-Culture
Two co-culture models as matured neuronal cells derived from NE-4C cells and BV-2 microglial cells, and undifferentiated NE-4C neural stem cells and BV-2 microglial cells prepared as mentioned above were used to investigate whether KP extracts could suppress Aβ42-induced inflammation via microglial activation. Aβ42 (1 µM) and extracts including KP1, KP2, and KP3 (0.5, 1, 2, 4, and 8 µg/mL) were added to the culture system medium for 24 h. The culture supernatant was collected and measured for IL-6 and nitrite levels. BV-2 cells were collected to evaluate the expression of IL-6 and iNOS mRNA and determine intracellular ROS. Differentiated neuronal cells and undifferentiated NE-4C neural stem cells were collected to determine cytotoxicity and intracellular ROS.
2.9. Effects of KP Extracts on Neurogenesis of Aβ42-Induced NE-4C Cells in Monoculture
NE-4C cells were induced for differentiation in the supplemented neurobasal medium for 24 h and then exposed to Aβ42 (0.25 µM) with or without KP extracts at nontoxic concentrations (0.5, 1, 2, 4, and 8 µg/mL) for six days. The differentiation medium was replaced every 48 h. At day six, cells were dissociated, seeded onto 24-well culture plates and grown until day twelve. Differentiated neuronal cells were collected for expression of βIII-tubulin and MAP-2 analysis.
2.10. Effects of KP Extracts on Neurogenesis in Aβ42-Induced NE-4C Cells in Co-Culture with BV-2 Cells
The BV-2 cells were loaded for co-culture with plated NE-4C cells for one day in the supplemented neurobasal medium. Then, Aβ42 (0.25 µM) with or without extracts including KP1, KP2, and KP3 (0.5, 1, 2, 4, and 8 µg/mL) was added, with differentiation continued for five days. Culture media were collected for IL-6 and nitrite level determination. BV-2 cells were collected for quantitative PCR analysis of IL-6 and iNOS expression and to determine the intracellular ROS. NE-4C cells were dissociated, seeded onto 24-well culture plates, and grown until day twelve without Aβ42 or KP extracts. Differentiated neuronal cells were collected for βIII-tubulin and MAP-2 mRNA analysis.
2.11. Sodium 3′-[1-(Phenylaminocarbonyl)-3,4-tetrazolium]-bis (4-Methoxy-nitro) Benzene Sulfonic Acid Hydrate (XTT) Reduction Assay
The cytotoxicity using an XTT-based assay was performed as previously detailed [29,30]. After the exposure times, the media was discarded. Then, 0.3 mg/mL XTT and 125 mM PMS were added. After incubation at 37 °C for 2 h, the absorbance was measured at 450 nm (SpectraMax® iD3, Molecular Devices, San Jose, CA, USA). Results are expressed as a percentage of cells that were untreated as a negative control.
2.12. Determination of ATP Levels
The total intracellular ATP level was determined using the CellTiter-Glo® Luminescence assay kit. Following cell treatment, the assay reagent containing substrate was added per each well and mixed for 30 min under light protection. The luminescence at 550 nm was measured (SpectraMax® iD3, Molecular Devices, San Jose, CA, USA), and the luminescence signal was expressed as a percentage of control.
2.13. Interleukin-6 (IL-6) Measurement
After the treatment, the culture medium was harvested and centrifuged at 2000× g for 10 min at 4 °C and kept at −20 °C until analysis. The culture supernatant was submitted to an IL-6 quantitative sandwich ELISA kit. The absorbance was measured at 450 nm using a microplate reader. The concentration of IL-6 in the samples was calculated in the comparison with the standard, curve (ranging from 10 to 500 pg/mL of IL-6).
2.14. Nitric Oxide (NO) Measurement
Griess reagent was used to NO levels. The culture medium (50 µL) was mixed with sulfanilamide solution (50 µL) for 10 min before being incubated with napthylethylenediamine dihydrochloride solution (50 µL) for 10 min. The absorbance at 520 nm was determined by a microplate reader.
2.15. Intercellular Reactive Oxygen Species (ROS) Measurement
The treated cells were washed with PBS, followed by preincubation with 20 µM 2,7-dichlorofluorescein diacetate (DCFDA) in a prewarmed culture medium for 30 min at 37 °C in the dark. The supernatant was removed, and the cells were washed with PBS, followed by the addition of 200 μL of cell lysis buffer (90% dimethyl sulfoxide/10% PBS). The mixtures were incubated for 5 min. The mixture (150 μL) was then transferred to a black 96-well plate, and the fluorescence was quantitated using a fluorometric plate reader (SpectraMax® iD3, Molecular Devices, San Jose, CA, USA) at 480 nm/530 nm excitation/emission wavelengths [36].
2.16. Reverse Transcription–Quantitative Polymerase Chain Reaction (RT–qPCR)
Total RNA was extracted from the treated cells using Tri-RNA Reagent. The RNA quality and quantity were analyzed using Nanodrop ND-1000 spectrophotometry (NanoDrop Technologies, Wilmington, DE, USA). One hundred nanograms of total RNA was added and prepared in the RT–qPCR reaction mixture, qPCRBIO SyGreen 1-step Lo-ROX. Quantitative polymerase chain reaction (qPCR) was performed using qTOWER3 Real-Time PCR Systems (Analytik Jena, Langewiesen, Germany) and then analyzed using qPCRsoft 3.4 software (Analytik Jena, Langewiesen, Germany). Relative levels of target gene expression were normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) mRNA using the 2−ΔΔCT method [37]. Each experiment was replicated at least three times, and the primer sequences are listed in the Supplementary Table S1.
2.17. Statistical Analysis
Data from at least three separate experiments are reported as mean ± standard deviation (SD). GraphPad Prism 9.0 (GraphPad Software, Boston, MA, USA) was used to statistically analyze the data. A one-way ANOVA followed by Tukey’s test was used to determine the statistical significance of differences between groups. A probability of 0.05 or less (p ≤ 0.05) was considered statistically significant.
3. Results
3.1. KP Extraction and Phytochemical Analysis
KP extraction yielded ethanolic crude extract (KP1), hexane (KP2), chloroform (KP3), ethyl acetate (KP4), and residue (KP5) fractions as 4.67%, 18.6%, 78.0%, 0.49%, and 0.67%, respectively. KP3 extracted from chloroform contained major K. parviflora components, suggesting the predominance of nonpolar phytochemicals. HPLC analysis detected caffeic acid and rutin in KP4, whereas only catechin was found in KP5 (Table 1 and Supplementary Figure S1). The flavone contents of KP have been well-documented and methoxyflavone derivatives were also determined, as shown in Table 1 and Supplementary Figure S2. DMF, PMF, and TMF were predominant in KP1 and KP3, with minor amounts in KP2 and KP4. TMF was the most abundant flavone derivative found in KP1 to KP4 compared to DMF and TMF. Interestingly, these three derivatives were not detectable in the KP5 fraction.
Table 1.
Quantification of phytochemicals, DMF, PMF, and TMF contents in K. parviflora crude extract and its fractions.
| Detected Compounds (mg/g Extract) |
Crude Ethanolic Extract (KP1) |
KP Fractions | |||
|---|---|---|---|---|---|
| Hexane (KP2) |
Chloroform (KP3) |
Ethyl Acetate (KP4) |
Residue (KP5) |
||
| Caffeic acid | ND | ND | ND | 4.07 ± 0.91 | ND |
| Catechin | ND | ND | ND | ND | 15.6 ± 1.40 |
| Rutin | ND | ND | ND | 13.4 ± 2.80 | ND |
| DMF | 103.27 ± 1.15 | 8.75 ± 0.17 | 132.40 ± 0.01 | 2.90 ± 0.05 | ND |
| PMF | 123.95 ± 38.83 | 2.74 ± 0.34 | 156.64 ± 0.14 | 23.34 ± 0.02 | ND |
| TMF | 517.19 ± 2.10 | 35.19 ± 3.31 | 649.54 ± 6.67 | 76.09 ± 0.26 | ND |
The value is represented as mean ± SD. ND.: non-detectable. DMF: 5,7-dimethoxyflavone, PMF: 3,5,7,3′,4′-pentamethoxyflavone, and TMF: 5,7,4′-trimethoxyflavone.
3.2. Cytotoxicity Determination of Aβ42 and K. parviflora Extracts
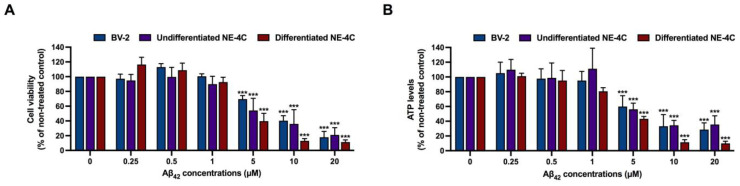
To investigate the protective effects of K. parviflora extract against Aβ42 peptides, we first determined the cytotoxic effects of Aβ42 peptides and the five fractions of K. parviflora extracts to establish the ranges of appropriate concentrations for the whole study, using XTT reduction and ATP levels as two independent cytotoxicity assays. The three cell lines, BV-2, undifferentiated NE-4C and differentiated NE-4C were treated with various ranges of Aβ42 and the five fractions of K. parviflora extracts (KP1–KP5) for 24 h. Figure 1A,B show that after 24 h of treatment, Aβ42 at ≤1 µM was not cytotoxic to all tested cells, while Aβ42 at ≥5 µM showed significant cytotoxicity toward all tested cells in our condition.
Figure 1.
Cytotoxicity effects of amyloid beta peptides (Aβ42) on BV-2 cells, undifferentiated NE-4C and differentiated NE-4C. (A) cytotoxicity effects of Aβ42 measured by XTT assay and (B) cytotoxicity effects of Aβ42 measured by ATP assay. The values are mean ± SD of three independent experiments and statistical significance was analyzed against untreated control (0 µM) of each cell line using one-way ANOVA followed by Tukey’s test. ***, p < 0.001.
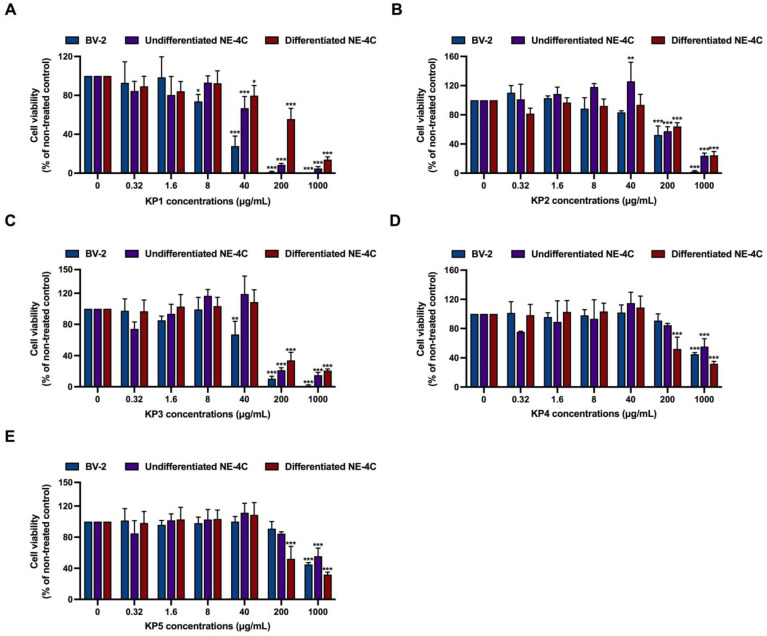
Results from the XTT assay also revealed that all five fractions of K. parviflora extracts (KP1–KP5) displayed different cytotoxic effects. In BV-2 cells, KP1 markedly showed toxicity starting from 40 µg/mL, while KP2–KP3 showed toxicity starting from 200 µg/mL and KP4–KP5 exhibited clear toxicity starting from 1000 µg/mL (Figure 2A–E). In undifferentiated NE-4C, KP1–KP3 showed toxicity starting from 200 µg/mL, and KP4–KP5 showed toxicity starting from 1000 µg/mL. Moreover, in differentiated NE-4C, significant toxicity was obtained from 200 µg/mL of KP1–KP5. Thus, 0.5 to 8 µg/mL of KP1–KP5, which were sub-cytotoxic concentrations, were selected for further investigation.
Figure 2.
Cytotoxicity effects of K. parviflora extracts (KP1–KP5) on BV-2 cells, undifferentiated NE-4C and differentiated NE-4C measured by XTT assay. (A) KP1 fraction, (B) KP2 fraction, (C) KP3 fraction, (D) KP4 fraction, and (E) KP5 fraction. The values are mean ± SD of three independent experiments and statistical significance was analyzed against untreated control (0 µM) of each cell line by one-way ANOVA followed by Tukey’s test. *, p < 0.05, **, p < 0.01, ***, p < 0.001.
3.3. Protective Effects of K. parviflora Extracts on Aβ42-Mediated Neurotoxicity
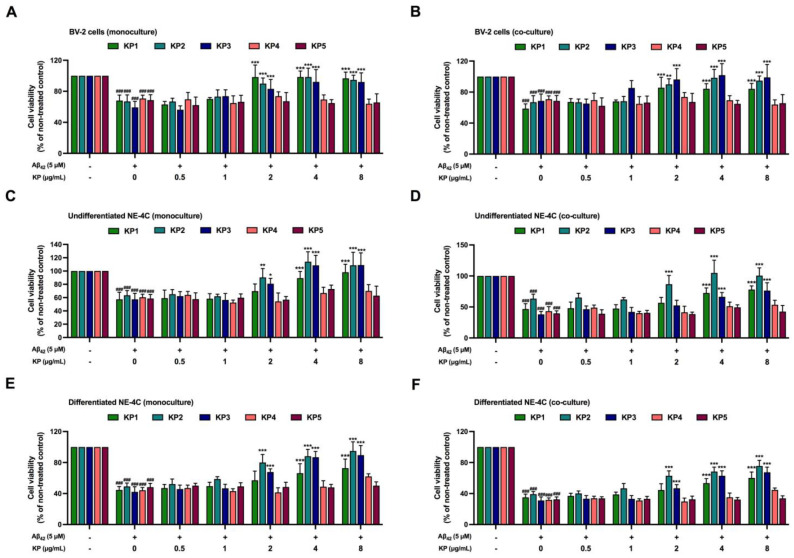
The protective effects of all five K. parviflora extract against Aβ42-mediated neurotoxicity in both NE-4C monoculture and co-culture between NE-4C and BV-2 cells were evaluated. Aβ42 at 5 µM was selected to induce neuronal death as this mirrored the IC50 value, using subtoxic doses of K. parviflora extracts (0.5–8 µg/mL). The NE-4C monoculture or co-culture between NE-4C and BV-2 cells was treated with Aβ42 and K. parviflora extracts. After 24 h of treatment, cytotoxicity was tested using the XTT assay. Figure 3A,B show that low concentrations of all five K. parviflora fractions (0.5–1 µg/mL) had no protective effects against Aβ42, while only KP1, KP2, and KP3 at 2–8 µg/mL effectively normalized Aβ42-mediated neurotoxicity in both monoculture and co-culture of BV-2 cells. Interestingly, KP1 (at least 4 µg/mL), KP2 (at least 2 µg/mL), and KP3 (at least 4 µg/mL), but not KP4 and KP5 significantly suppressed Aβ42 toxicity in undifferentiated and differentiated NE-4C cells (both monoculture and co-culture). Therefore, KP1, KP2, and KP3 fractions exhibited protective effects against Aβ42-induced neuronal death in both monoculture and co-culture models.
Figure 3.
Protective effects of five K. parviflora extracts against Aβ42-induced neuronal cell death in both NE-4C monoculture and co-culture between NE-4C and BV-2 cells determined using an XTT assay. (A) BV-2 monoculture, (B) BV-2 co-culture, (C) undifferentiated, NE-4C monoculture, (D) undifferentiated NE-4C co-culture, (E) differentiated NE-4C monoculture, and (F) differentiated NE-4C co-culture. Values are mean ± SD of three independent experiments. The ### indicates a significant difference between the control and cells exposed to Aβ42 peptides without KP extract at p < 0.001 using one-way ANOVA followed by Tukey’s test. The * indicates a significant difference between cells exposed to Aβ42 peptides without KP extract compared to cells exposed to Aβ42 peptides and various concentrations of KP extract (0.5–8 µg/mL) using one-way ANOVA followed by Tukey’s test. *, p < 0.05, **, p < 0.01, ***, p < 0.001.
3.4. Suppression of Aβ42-Induced Inflammation and Oxidative Stress by K. parviflora Extracts in BV-2 Cell Monoculture
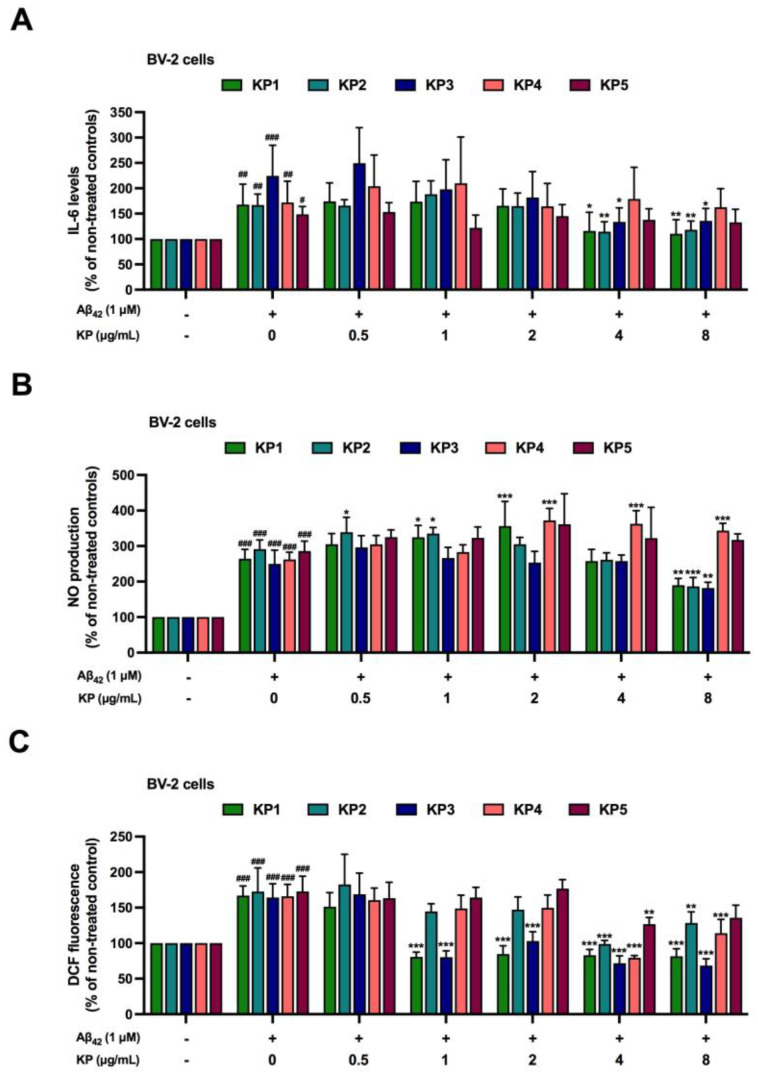
Aβ42 leads to neuronal death by two mechanisms as induction of inflammation and oxidative stress [38,39]. Thus, to explore how K. parviflora extracts prevented BV-2 death from Aβ42 (Figure 3A,B), we investigated the anti-inflammatory and antioxidant properties of KP1–KP5. BV-2 cell monoculture was co-exposed with Aβ42 (1 µM) with or without each fraction of K. parviflora extract (KP1-KP5). To avoid excessive inflammation and apoptotic interference, we used Aβ42 at 1 µM because this was a subtoxic dose (not more than IC20, Figure 1A,B). Biomarker levels of inflammatory responses such as interleukin-6 (IL-6) and nitrite, and cellular ROS were evaluated. Figure 4A–C confirm previous findings that Aβ42, even at a subtoxic dose (1 µM), operated as a neurotoxic agent by inducing inflammation (Figure 4A,B) and oxidative stress (Figure 4C) [38,39]. Treatment with K. parviflora extract (KP1-KP5) showed that only KP1, KP2, and KP3 fractions significantly reduced IL-6 levels (4 and 8 µg/mL), nitrite (8 µg/mL), and DCF fluorescence levels (KP1 and KP3 from 1–8 µg/mL and KP2 at 4 and 8 µg/mL) in a monoculture of Aβ42-treated BV-2 cells. Therefore, KP1, KP2, and KP3 prevented Aβ42-induced microglial death due to their anti-inflammatory and antioxidant properties.
Figure 4.
Anti-inflammatory and antioxidant effects of five K. parviflora fractions on Aβ42-induced neurotoxicity in monoculture of BV-2 cells. (A) IL-6 levels, (B) nitric oxide (NO) production, and (C) DCF fluorescence. Values are mean ± SD of three independent experiments. The # shows a significant difference between the control and cells exposed to Aβ42 peptides without KP extract using one-way ANOVA followed by Tukey’s test. #, p < 0.05, ##, p < 0.01, ###, p < 0.001. The * shows a significant difference between cells exposed to Aβ42 peptides without KP extract compared to cells exposed to Aβ42 peptides and various concentrations of KP extract (0.5–8 µg/mL) using one-way ANOVA followed by Tukey’s test. *, p < 0.05, **, p < 0.01, ***, p < 0.001.
3.5. Suppression of Aβ42-Induced Inflammation and Oxidative Stress by K. parviflora Extracts in Co-Culture between Differentiated NE-4C and BV-2 Cells
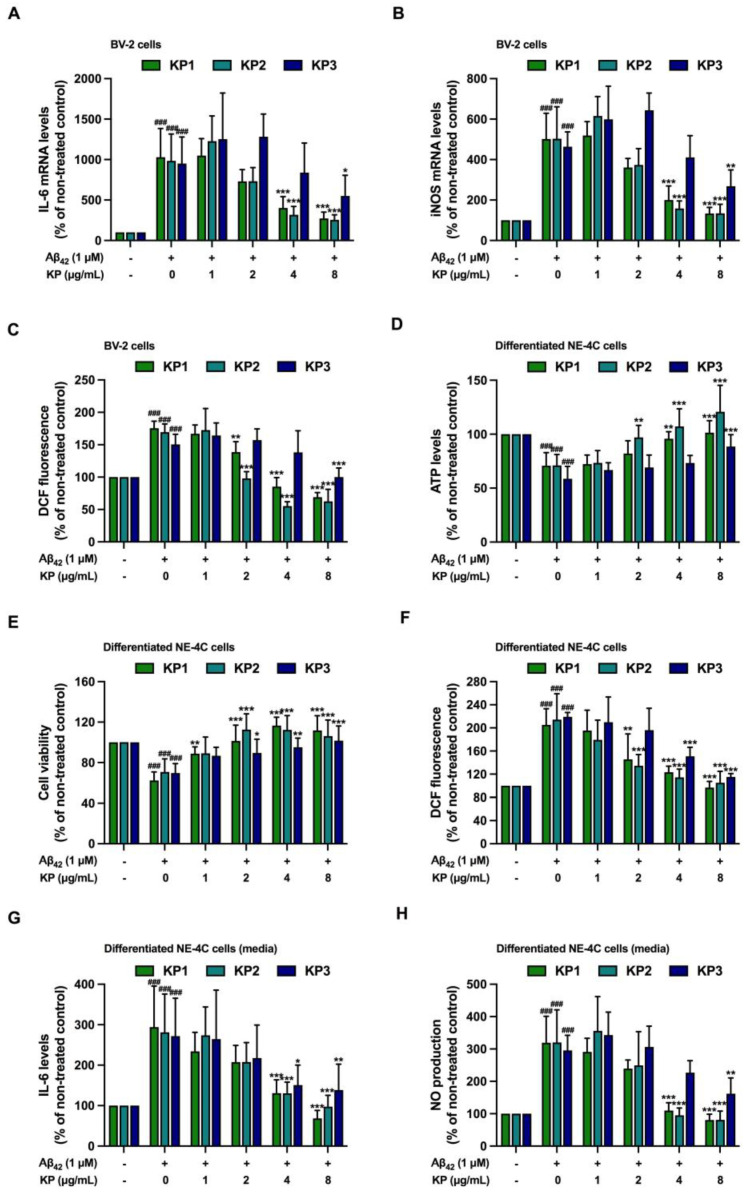
Inflamed microglia eventually lead to neuronal dysfunction and death. Figure 3 and Figure 4 show the protective effects of KP1, KP2, and KP3 against neuronal death, inflammation, and oxidative stress in microglial BV-2 cell monoculture. Thus, the anti-inflammation and antioxidative stress of KP1, KP2, and KP3 fractions were further studied in co-culture between differentiated NE-4C and BV-2 cells. IL-6 and inducible nitric oxide synthase (iNOS) mRNA levels and cellular ROS levels in BV2-cells were significantly induced after Aβ42 treatment, confirming the neurotoxic effect of Aβ42 peptides (Figure 5A–C). However, these markers were reduced when KP1 and KP2 at 4–8 µg/mL and KP3 at 8 µg/mL were applied.
Figure 5.
Anti-inflammatory and antioxidant effects of five K. parviflora fractions on Aβ42-induced neurotoxicity in co-culture between differentiated NE-4C and BV-2 cells. (A) IL-6 mRNA levels of BV-2 cells, (B) iNOS mRNA levels of BV-2 cells, (C) DCF fluorescence of BV-2 cells, (D) ATP levels of differentiated NE-4C, (E) cell viability of differentiated NE-4C, (F) DCF fluorescence of differentiated NE-4C, (G) IL-6 levels in culture media, and (H) NO production in the culture media. Values are mean ± SD of three independent experiments. The ### shows significant difference between the control and cells exposed to Aβ42 peptides without KP extract at p < 0.001 using one-way ANOVA followed by Tukey’s test. The * shows significant difference between cells exposed to Aβ42 peptides without KP extract compared to cells exposed to Aβ42 peptides and various concentrations of KP extract (1–8 µg/mL) using one-way ANOVA followed by Tukey’s test. *, p < 0.05, **, p < 0.01, ***, p < 0.001.
The effects of Aβ42 peptides on differentiated NE-4C cells were also investigated. In the co-culture, Aβ42 peptides markedly decreased cell viability and caused oxidative stress in differentiated NE-4C cells compared to the untreated control (Figure 5D–F), suggesting the occurrence of inflamed microglia-mediated neuronal death. KP1 and KP2 (2, 4, and 8 µg/mL) and KP3 (4 and 8 µg/mL) prevented neuronal death and oxidative stress in differentiated NE-4C cells. Figure 5G,H show that IL-6 and nitrite levels in the cell culture medium were dramatically induced by Aβ42 peptides, whereas levels were significantly reduced by KP1, KP2, and KP3 treatments. Therefore, Aβ42 peptides caused inflammation and oxidative stress in co-culture between microglia and differentiated NE-4C cells leading to neuronal death. Interestingly, KP fractions KP1, KP2, and KP3 significantly decreased inflamed microglia and improved neuronal viability under Aβ42 induction.
3.6. Suppression of Aβ42-Induced Inflammation and Oxidative Stress by K. parviflora Extracts in Co-Culture between Undifferentiated NE-4C and BV-2 Cells
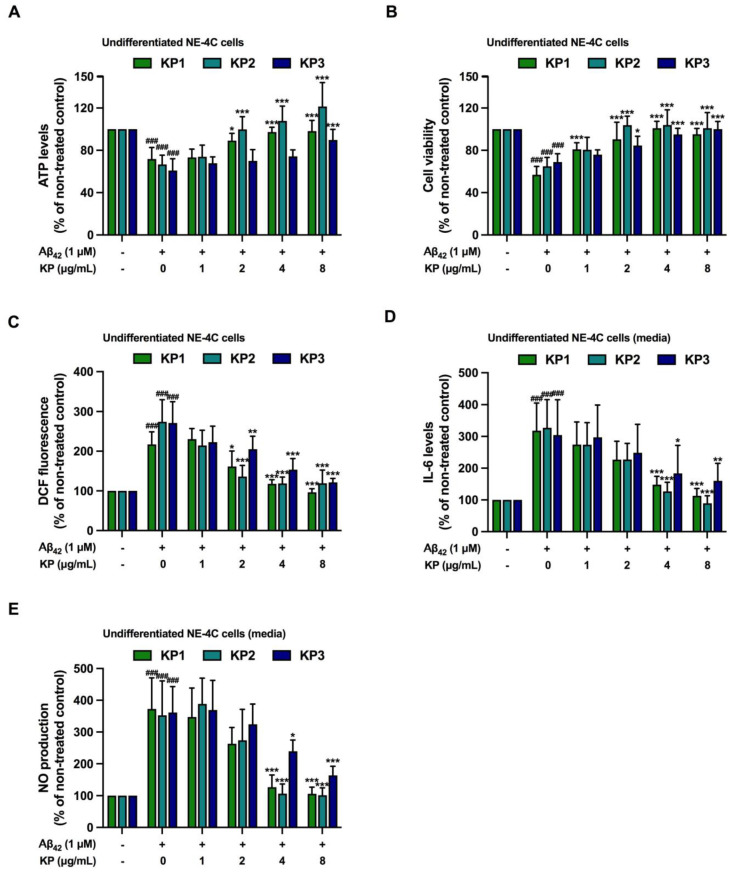
Figure 5 shows the effects of inflamed microglia on differentiated NE-4C death; however, the effects of inflammatory microglia on neural stem cells that eventually develop into functional neurons remain unclear. Hence, the results of Aβ42-mediated inflamed microglia on undifferentiated NE-4C cells using the same co-culture strategy were further elucidated.
Figure 5A–C illustrate that Aβ42 peptides activated IL6 and iNOS mRNA expressions and oxidative stress (DCF fluorescence), while all inflammatory and oxidative stress markers were quenched by KP1, KP2, and KP3 extracts. Interestingly, inflamed BV-2 cells also decreased cell viability (Figure 6A,B), and caused oxidative stress (Figure 6C) and inflammation (Figure 6D,E) of undifferentiated neural stem cells in the same manner as differentiated neurons (Figure 5D–F). Treatment with KP extracts (KP1-KP3) protected against neuronal death and decreased oxidative stress (Figure 6A–E). These data implied that (i) Aβ42-mediated inflamed microglia led to the cytotoxicity of differentiated neurons and also undifferentiated neural stem cells, and (ii) KP1–KP3 decreased inflamed microglia, which subsequently resulted in improved viability of undifferentiated neural stem cells.
Figure 6.
Anti-inflammatory and antioxidant effects of five K. parviflora fractions on Aβ42-induced neurotoxicity in co-culture between undifferentiated NE-4C and BV-2 cells. (A) ATP levels of undifferentiated NE-4C, (B) cell viability of undifferentiated NE-4C, (C) DCF fluorescence of undifferentiated NE-4C, (D) IL-6 levels in culture media, and (E) NO production in the culture media. Values are mean ± SD of three independent experiments. The ### shows a significant difference between the control and cells exposed to Aβ42 peptides without KP extract at p < 0.001 using one-way ANOVA followed by Tukey’s test. The * shows a significant difference between cells exposed to Aβ42 peptides without KP extract compared to cells exposed to Aβ42 peptides and various concentrations of KP extract (1–8 µg/mL) using one-way ANOVA followed by Tukey’s test. *, p < 0.05, **, p < 0.01, ***, p < 0.001.
3.7. Protective Effects of K. parviflora Extracts on Neurogenesis of Aβ42-Treated NE-4 Cells in Monoculture
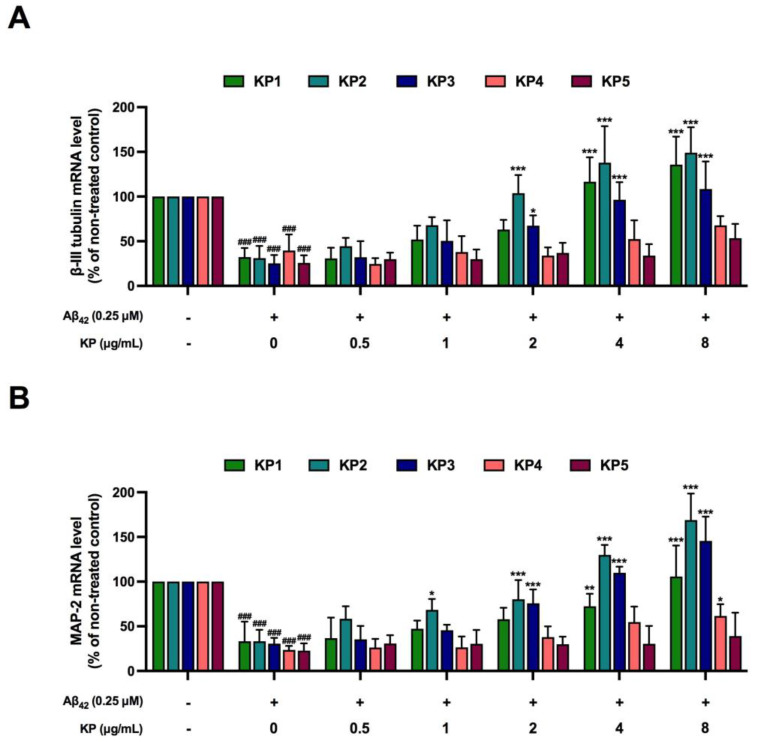
Data in Figure 6 show that Aβ42 peptides reduced the viability of neural stem cells. Protective effects of KP extracts on neurogenesis-inhibiting Aβ42 peptide-treated cells were further elucidated by studying the expressions of two neuron-specific proteins, namely class III β tubulin (beta-III tubulin) and microtubule-associated protein 2 (MAP-2) in monoculture of differentiated NE-4 cells. Reductions in both beta-III tubulin and MAP2 mRNA levels were obtained when differentiated NE-4 cells were exposed to Aβ42 peptides (Figure 7A,B), suggesting that Aβ42 peptides directly inhibit neurogenesis, even at a nontoxic dose (0.25 µM, Figure 1A,B). Cells receiving both Aβ42 and various concentrations of KP fractions (KP2 and KP3 starting at 2 µg/mL, while KP1 starting at 4 µg/mL) showed significant recovery of beta-III tubulin and MAP-2 mRNA expressions, albeit at different potency. KP1, KP2, and KP3 fractions showed promising protective effects against neurogenesis-inhibiting Aβ42 peptides in the monoculture of differentiated NE-4 cells (Figure 7A,B).
Figure 7.
The protective effects of K. parviflora against neurogenesis-inhibiting Aβ42 peptides in monoculture of differentiated NE-4C cells. (A) beta-III tubulin mRNA levels and (B) MAP-2 mRNA levels. The ### shows a significantly different between control and cells exposed to Aβ42 peptides without KP extract at p < 0.001 using one-way ANOVA followed by Tukey’s test. The * shows a significantly different between cells exposed to Aβ42 peptides without KP extract compared to cells exposed to Aβ42 peptides and various concentrations of KP extract (0.5–8 µg/mL) using one-way ANOVA followed by Tukey’s test. *, p < 0.05, **, p < 0.01, ***, p < 0.001.
3.8. Protective Effects of K. parviflora Extracts Neurogenesis in Aβ42-Induced Differentiated NE-4C in Co-Culture with BV-2 Cells
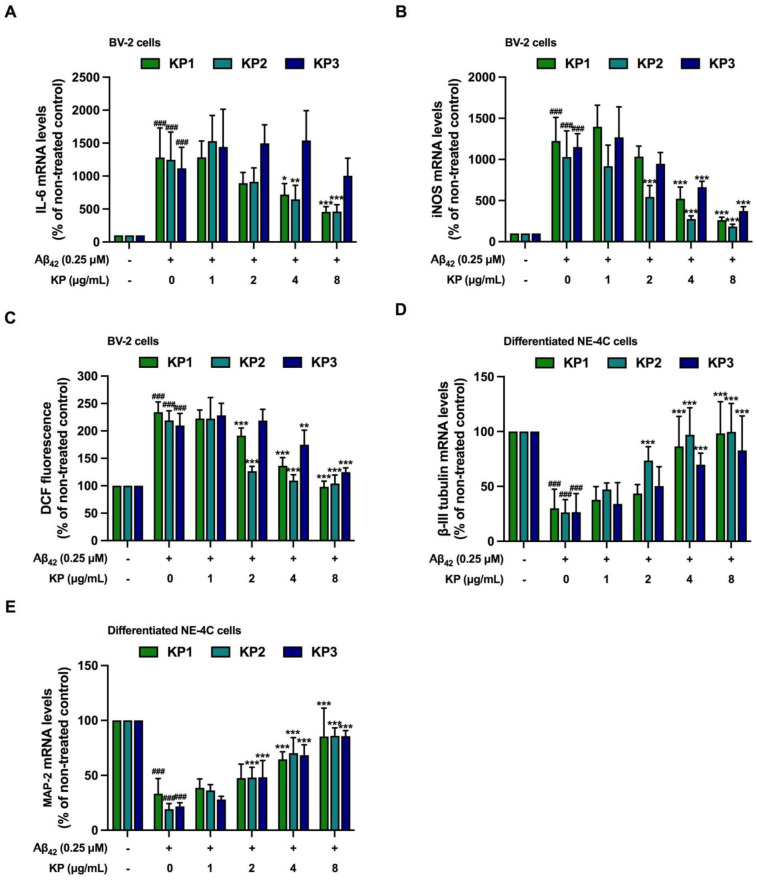
In addition to monoculture (Figure 7), we further investigated the protective effects of K. parviflora extracts against neurogenesis-inhibiting Aβ42 peptides in co-culture between differentiated NE-4C and BV-2 cells. Figure 8A–C show that without KP extracts, Aβ42 peptides induced inflammation and oxidative stress in BV-2 cells, similar to previous data (Figure 5A–C). Reduced inflammation and oxidative stress were also observed when each KP extract was added. Consistent with results obtained from undifferentiated cells shown in Figure 7, differentiated NE-4C cells exposed to Aβ42 peptides showed a reduction of both beta-III tubulin and MAP-2 mRNA expressions in co-culture (Figure 8D,E), suggesting that Aβ42 peptides not only inhibited neurogenesis directly but also reduced neurogenesis indirectly through inflamed microglia.
Figure 8.
The protective effects of K. parviflora on neurogenesis in the co-culture between differentiated NE-4C and BV-2 cells treated with Aβ42. (A) IL-6 mRNA levels of BV-2 cells, (B) relative iNOS mRNA levels of BV-2 cells, (C) DCF fluorescence of BV-2 cells, (D) beta-III tubulin mRNA levels of differentiated NE-4C cells, and (E) MAP-2 mRNA levels of differentiated NE-4C cells. The values are mean ± SD of three independent experiments. The ### shows a significantly different between control and cells exposed to Aβ42 peptides without KP extract at p < 0.001 using one-way ANOVA followed by Tukey’s test. The * shows a significantly different between cells exposed to Aβ42 peptides without KP extract compared to cells exposed to Aβ42 peptides and various concentrations of KP extract (1–8 µg/mL) using one-way ANOVA followed by Tukey’s test. *, p < 0.05, **, p < 0.01, ***, p < 0.001.
Protective effects of the promising KP extracts KP1, KP2, and KP3 against neurogenesis-inhibiting Aβ42 peptides were also investigated. Intriguingly, protection indicated by the induction of beta-III tubulin and MAP-2 mRNA expressions was observed in all three KP fractions at 4 and 8 µg/mL (Figure 8D,E). These data indicated that KP1, KP2, and KP3 improved neurogenesis against Aβ42 peptide treatment.
4. Discussion
Alzheimer’s disease (AD) induces gradual cognitive impairment, with major pathological features such as the deposition of extracellular amyloid beta (Aβ) plaques and intercellular neurofibrillary tangles of tau protein. Microglial activation and neuroinflammation by Aβ plaques have been proposed as underlying and connecting components in the development of AD [2,3,4,40]. The involvement of stem cells in adult neurogenesis is critical for memory and cognitive performance [41]. In AD cases, Aβ plaque impairs the proliferation and differentiation of both neural stem cells and neurons [5,6,7] due to excessive induction of neuronal death and oxidative stress [6,7]. Therefore, limiting this vicious cycle by suppressing Aβ toxicity and promoting differentiation and/or proliferation of neural stem cells by safety agents shows promise as a potential therapeutic strategy to delay AD progression. K. parviflora has shown promising neuroprotective activity in several previous studies [42,43,44]. An ethanolic extract of K. parviflora protected glutamate-induced cell injury in mouse hippocampal neuronal cells [43], while K. parviflora protected oxidative stress-related brain damage and memory deficit induced by focal cerebral ischemia in rats [44]. The anti-AD effects targeting AChE and Aβ formation of K. parviflora have been previously studied [45,46] but the protective impacts of K. parviflora on Aβ-induced neurotoxicity, neuroinflammation, and neurogenesis have not yet been examined. In this study, NE-4C neural stem cells (undifferentiated), BV-2 microglia, and NE-4C-derived neurons (differentiated) were used in both monoculture and co-culture of NE-4C neural stem cells and BV-2 microglia, and co-cultures of NE-4C derived neurons and BV-2 microglia, to assess the impact of K. parviflora fractions on neuroprotective and anti-neuroinflammatory Aβ42 challenges, and on neurogenesis processes compromised by Aβ42.
The K. parviflora fractions were characterized as crude ethanolic (KP1), n-hexane (KP2), chloroform (KP3), ethyl acetate (KP4) and residue (KP5). 5,7-Dimethoxyflavone (DMF) was detectable in KP3, KP1, KP2, and KP4, while 3,5,7,3’,4’-pentamethoxyflavone (PMF) and 5,7,4’-trimethoxyflavone (TMF) were found in KP1, KP3, and KP4. DMF was the primary bioactive phytochemical with antioxidant and anti-inflammatory activities [47,48,49,50,51]. DMF is used as a quality control marker, with KP powder produced for food ingredients containing at least 2.5% [52]. Our crude extract of KP contained 10.3% more DMF than the recommendation, while DMF, TMF, and PMF were absorbed through the GI tract and distributed throughout the brain, suggesting that these methoxyflavones, especially DMF, may offer neuroprotection [53,54] by inhibiting oxidative stress and neuroinflammation.
Aβ42 generated cytotoxicity and elevated intracellular ROS in NE-4C neural stem cells and NE-4C derived neurons, while toxicity was greatly amplified when these cells were co-cultured with microglial cells. Co-exposure of Aβ42 at 5 μM with each KP fraction showed that crude ethanolic (KP1), n-hexane (KP2), and chloroform (KP3) fractions significantly decreased toxicity in a concentration-dependent manner in both mono- and co-cultures of BV-2, neural NE-4C stem cells, and neuronal-derived NE-4C cells (Figure 3). KP extract had neuroprotective impacts on mice hippocampus neuronal cells by promoting brain-derived neurotrophic factor (BDNF) expression and the extracellular signal-regulated kinase pathway (ERK) [43]. Furthermore, these three fractions demonstrated anti-inflammation triggered by Aβ42 in both BV-2 monoculture and co-culture (Figure 4, Figure 5 and Figure 6), with attenuated IL-6 and NO levels in the culture medium. These bioactive fractions reduced microglial activation by decreasing inducible nitric oxide synthase (iNOS) mRNA expression and intracellular ROS. NE-4C and neuron cells co-cultured with microglia were also protected by K. parviflora fractions from Aβ42, with boosted ATP levels and cell viability, while reducing intracellular ROS. K. parviflora has been shown to have anti-inflammatory properties that suppress the production of IL-6 in lipopolysaccharide-stimulated monocytes by inhibiting the activation of ERK1/2 and protein kinase B (Akt) [14,15]. Taken together, K. parviflora KP1, KP2, and KP3 fractions containing DMF protected toxicity in BV-2, neural NE-4C stem cells, and neuronal-derived NE-4C cells induced by Aβ42 peptides, while also protecting against cellular damage induced by microglial activation, as characteristics attributed to DMF [49,50,55].
To study the impact of KP extracts on Aβ42-disrupted neurogenesis, a low concentration of Aβ42 (0.25 µM) together with KP extract was applied in a monoculture of NE-4C cells for six days during 12 days of differentiation (Figure 7). The stated exposure period aimed was to assess how Aβ42 and the extracts impacted early differentiation before neuronal maturation [56]. The three KP fractions (KP1, KP2, and KP3) inhibited the action of Aβ42 and reversed the expression of specific neuronal mRNA markers, βIII-tubulin and MAP-2. The extracts reduced Aβ42-driven IL-6 and iNOS mRNA levels as well as intracellular ROS in microglial cells, coupled with lower IL-6 and NO levels in the coculture medium (Figure 8). Concerning differentiated neurons from NE-4C cells cocultured with microglia cells, the extracts also restored impaired neuronal development from Aβ42 in a similar way to the NE-4C cell monoculture. No data exist to demonstrate the influence of K. parviflora on neural stem cell differentiation but K. parviflora protected the rat brain against valproic acid-induced impairments in spatial memory and neural stem cell proliferation in the dentate gyrus [20]. These findings showed that K. parviflora extracts prevented the influence of Aβ42 on neurogenesis both directly on the functioning of NE-4C cells and indirectly by suppressing microglia-induced neuroinflammation. Apart from antioxidative and anti-inflammatory activities, neurogenesis signal transduction pathways involve ERK1/2, protein kinase A (PKA), Akt, WNT/β-catenin, protein kinase C (PKC), and BDNF [10,12]. Further research into molecular targets and the many biological neurogenetic activities of K. parviflora should concentrate on the ERK1/2, PKA, Akt, and BDNF signaling pathways [15,43,57].
The biological and toxic properties of methoxyflavones have generally focused on DMF. This study examined and compared the anti-inflammation and cytotoxicity of DMF, TMF and PMF in human dermal fibroblasts. TMF exhibited the highest cytotoxicity, while PMF did not influence on cell viability. TMF was the most effective polymethoxyflavone in decreasing TNF-α induced fibroblast damage through the MAPK and NF-κB pathways [58], while PMF was nontoxic and protective against oxidative DNA damage in RAW 264.7 macrophages [59]. Limited evidence exists on the anti-neuroinflammation, neuroprotection, and neurogenesis of DMF, TMF, and PMF individually. Current research is unable to distinguish their activity in each KP fraction or compare their efficacy. Further research is required to determine which methoxyflavone contributes to neuroinflammation and neurogenesis. Moreover, whether the neuroprotective mechanism of the KP extract and its active compound(s) is related to a decrease in ROS or other off-target effects should be investigated further.
5. Conclusions
Our results revealed that K. parviflora extracts protected against Aβ42 induced cellular damage of NE-4C neural stem cells, BV-2 microglia, and NE-4C derived neurons and also reduced microglia-induced cellular damage in NE-4C neural stem cells and neurons by Aβ42. Moreover, K. parviflora extracts also inhibited the inhibitory effects of Aβ42 on neuronal differentiation. These findings provide clues about the role of K. parviflora extracts in neuroprotection and neurogenesis and curing Aβ42-dependent AD. However, further detailed investigations are required to explore the molecular pathways governing neuroprotective actions against Aβ42 and the stimulatory effects in the neurogenesis of K. parviflora extracts.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nu15051098/s1. Supplementary Table S1. Primers used for real-time PCR; Supplementary Figure S1. HPLC chromatogram for determination of catechin, caffeic acid, rutin, rosmarinic acid, quercetin, apigenin and kaempferol in (A) crude ethanolic extract (KP1), and (B) hexane (KP2), (C) chloroform (KP3), (D) ethyl acetate (KP4), and (E) residue (KP5) fractions, (F) shows the HPLC fingerprint of a standard mixture including, (A) catechin, (B) caffeic acid, (C) rutin, (D) rosmarinic acid, (E) quercetin, (F) apigenin, (G) kaempferol, and (H) 5,7-dimethylflavone; Supplementary Figure S2. HPLC chromatogram for determination of flavone derivatives including PMF (3,5,7,3’,4’-pentamethoxyflavone), DMF (5,7-dimethoxyflavone) and TMF (5,7,4’-trimethoxyflavone) contents in (A) crude ethanolic extract (KP1), and (B) hexane (KP2), (C) chloroform (KP3), (D) ethyl acetate (KP4), and (E) residue (KP5) fractions, (F) shows the HPLC fingerprint of a standard mixture of PMF, DMF, and TMF.
Author Contributions
Conceptualization, P.T., P.P. and B.C.; methodology, A.C., P.P. and B.C.; validation, P.T., A.C., P.P. and B.C.; formal analysis, P.T., J.K., P.P. and B.C.; investigation, A.C., J.K., S.P. and W.I.; resources, S.B. and B.C.; data curation, A.C.; writing—original draft preparation, P.T., P.P. and B.C.; writing—review and editing, P.T., A.C., J.K., S.P., S.B., W.I., P.P. and B.C.; visualization, J.K. and W.I.; supervision, P.T., P.P. and B.C.; project administration, B.C.; funding acquisition, B.C. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was supported by grants from the Agricultural Research Development Agency (ARDA), Thailand (CRP6105020830 and CRP6505030570).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.WHO . Global Action Plan on the Public Health Response to Dementia 2017–2025. World Health Organization; Geneva, Switzerland: 2017. [Google Scholar]
- 2.Hu J., Wang X. Alzheimer’s Disease: From Pathogenesis to Mesenchymal Stem Cell Therapy—Bridging the Missing Link. Front. Cell. Neurosci. 2021;15:811852. doi: 10.3389/fncel.2021.811852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murphy M.P., LeVine H., 3rd Alzheimer’s disease and the amyloid-beta peptide. J. Alzheimers Dis. 2010;19:311–323. doi: 10.3233/JAD-2010-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hemonnot A.L., Hua J., Ulmann L., Hirbec H. Microglia in Alzheimer Disease: Well-Known Targets and New Opportunities. Front. Aging Neurosci. 2019;11:233. doi: 10.3389/fnagi.2019.00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mansor N.I., Ntimi C.M., Abdul-Aziz N.M., Ling K.H., Adam A., Rosli R., Hassan Z., Nordin N. Asymptomatic neurotoxicity of amyloid β-peptides (Aβ1-42 and Aβ25-35) on mouse embryonic stem cell-derived neural cells. Bosn. J. Basic Med. Sci. 2021;21:98–110. doi: 10.17305/bjbms.2020.4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee J., Park H.-H., Koh S.-H., Hojin C. Neural Stem Cell Death Mechanisms Induced by Amyloid Beta. Dement. Neurocogn. Disord. 2017;16:121. doi: 10.12779/dnd.2017.16.4.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lu J., Li Y., Mollinari C., Garaci E., Merlo D., Pei G. Amyloid-β Oligomers-induced Mitochondrial DNA Repair Impairment Contributes to Altered Human Neural Stem Cell Differentiation. Curr. Alzheimer Res. 2019;16:934–949. doi: 10.2174/1567205016666191023104036. [DOI] [PubMed] [Google Scholar]
- 8.Li Puma D.D., Piacentini R., Grassi C. Does impairment of adult neurogenesis contribute to pathophysiology of alzheimer’s disease? A still open question. Front. Mol. Neurosci. 2021;13:578211. doi: 10.3389/fnmol.2020.578211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mimica N., Presecki P. Side effects of approved antidementives. Psychiatr. Danub. 2009;21:108–113. [PubMed] [Google Scholar]
- 10.Kim H.J. Regulation of Neural Stem Cell Fate by Natural Products. Biomol. Ther. 2019;27:15–24. doi: 10.4062/biomolther.2018.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saud B., Malla R., Shrestha K. A Review on the Effect of Plant Extract on Mesenchymal Stem Cell Proliferation and Differentiation. Stem. Cells Int. 2019;2019:7513404. doi: 10.1155/2019/7513404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang J., Hai J., Liu W., Luo Y., Chen K., Xin Y., Pan J., Hu Y., Gao Q., Xiao F., et al. Gallic Acid Induces Neural Stem Cell Differentiation into Neurons and Proliferation through the MAPK/ERK Pathway. J. Agric. Food Chem. 2021;69:12456–12464. doi: 10.1021/acs.jafc.1c04011. [DOI] [PubMed] [Google Scholar]
- 13.Lin K.L., Zhang J., Chung H.L., Wu X.Y., Liu B., Zhao B.X., Sze S.C., Zhou P.Z., Yung K.K., Zhang S.Q. Total Ginsenoside Extract from Panax ginseng Enhances Neural Stem Cell Proliferation and Neuronal Differentiation by Inactivating GSK-3β. Chin. J. Integr. Med. 2022;28:229–235. doi: 10.1007/s11655-021-3508-1. [DOI] [PubMed] [Google Scholar]
- 14.Takuathung M.N., Potikanond S., Sookkhee S., Mungkornasawakul P., Jearanaikulvanich T., Chinda K., Wikan N., Nimlamool W. Anti-psoriatic and anti-inflammatory effects of Kaempferia parviflora in keratinocytes and macrophage cells. Biomed Pharm. 2021;143:112229. doi: 10.1016/j.biopha.2021.112229. [DOI] [PubMed] [Google Scholar]
- 15.Thaklaewphan P., Ruttanapattanakul J., Monkaew S., Buatoom M., Sookkhee S., Nimlamool W., Potikanond S. Kaempferia parviflora extract inhibits TNF-α-induced release of MCP-1 in ovarian cancer cells through the suppression of NF-κB signaling. Biomed Pharm. 2021;141:111911. doi: 10.1016/j.biopha.2021.111911. [DOI] [PubMed] [Google Scholar]
- 16.Yoshino S., Awa R., Miyake Y., Fukuhara I., Sato H., Ashino T., Tomita S., Kuwahara H. Daily intake of Kaempferia parviflora extract decreases abdominal fat in overweight and preobese subjects: A randomized, double-blind, placebo-controlled clinical study. Diabetes Metab. Syndr. Obes. 2018;11:447–458. doi: 10.2147/DMSO.S169925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kobayashi H., Suzuki R., Sato K., Ogami T., Tomozawa H., Tsubata M., Ichinose K., Aburada M., Ochiai W., Sugiyama K., et al. Effect of Kaempferia parviflora extract on knee osteoarthritis. J. Nat. Med. 2018;72:136–144. doi: 10.1007/s11418-017-1121-6. [DOI] [PubMed] [Google Scholar]
- 18.Chen D., Li H., Li W., Feng S., Deng D. Kaempferia parviflora and Its Methoxyflavones: Chemistry and Biological Activities. Evid.-Based Complement Altern. Med. 2018;2018:4057456. doi: 10.1155/2018/4057456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sawasdee P., Sabphon C., Sitthiwongwanit D., Kokpol U. Anticholinesterase activity of 7-methoxyflavones isolated from Kaempferia parviflora . Phytother. Res. 2009;23:1792–1794. doi: 10.1002/ptr.2858. [DOI] [PubMed] [Google Scholar]
- 20.Welbat J.U., Chaisawang P., Chaijaroonkhanarak W., Prachaney P., Pannangrong W., Sripanidkulchai B., Wigmore P. Kaempferia parviflora extract ameliorates the cognitive impairments and the reduction in cell proliferation induced by valproic acid treatment in rats. Ann. Anat. 2016;206:7–13. doi: 10.1016/j.aanat.2016.04.029. [DOI] [PubMed] [Google Scholar]
- 21.Umka J., Mustafa S., ElBeltagy M., Thorpe A., Latif L., Bennett G., Wigmore P.M. Valproic acid reduces spatial working memory and cell proliferation in the hippocampus. Neuroscience. 2010;166:15–22. doi: 10.1016/j.neuroscience.2009.11.073. [DOI] [PubMed] [Google Scholar]
- 22.Mula M., Trimble M.R. Antiepileptic drug-induced cognitive adverse effects: Potential mechanisms and contributing factors. CNS Drugs. 2009;23:121–137. doi: 10.2165/00023210-200923020-00003. [DOI] [PubMed] [Google Scholar]
- 23.Yoshino S., Awa R., Ohto N., Miyake Y., Kuwahara H. Toxicological evaluation of standardized Kaempferia parviflora extract: Sub-chronic and mutagenicity studies. Toxicol. Rep. 2019;6:544–549. doi: 10.1016/j.toxrep.2019.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Felice F.G., Wu D., Lambert M.P., Fernandez S.J., Velasco P.T., Lacor P.N., Bigio E.H., Jerecic J., Acton P.J., Shughrue P.J., et al. Alzheimer’s disease-type neuronal tau hyperphosphorylation induced by Aβ oligomers. Neurobiol. Aging. 2008;29:1334–1347. doi: 10.1016/j.neurobiolaging.2007.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ruiz-Muñoz A.M., Nieto-Escamez F.A., Aznar S., Colomina M.T., Sanchez-Santed F. Cognitive and histological disturbances after chlorpyrifos exposure and chronic Aβ (1–42) infusions in Wistar rats. Neurotoxicology. 2011;32:836–844. doi: 10.1016/j.neuro.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 26.Stine W.B., Jungbauer L., Yu C., LaDu M.J. Preparing synthetic Aβ in different aggregation states. Methods Mol. Biol. 2011;670:13–32. doi: 10.1007/978-1-60761-744-0_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hawiset T., Muchimapura S., Wattanathorn J., Sripanidkulchai B. Screening Neuropharmacological Activities of Kaempferia parviflora (Krachai dam) in Healthy Adult Male Rats. Am. J. Appl. Sci. 2011;8:695–702. doi: 10.3844/ajassp.2011.695.702. [DOI] [Google Scholar]
- 28.Plaingam W., Sangsuthum S., Angkhasirisap W., Tencomnao T. Kaempferia parviflora rhizome extract and Myristica fragrans volatile oil increase the levels of monoamine neurotransmitters and impact the proteomic profiles in the rat hippocampus: Mechanistic insights into their neuroprotective effects. J. Tradit. Complement Med. 2017;7:538–552. doi: 10.1016/j.jtcme.2017.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Phochantachinda S., Chatchaisak D., Temviriyanukul P., Chansawang A., Pitchakarn P., Chantong B. Ethanolic Fruit Extract of Emblica officinalis Suppresses Neuroinflammation in Microglia and Promotes Neurite Outgrowth in Neuro2a Cells. Evid.-Based Complement. Altern. Med. 2021;2021:6405987. doi: 10.1155/2021/6405987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Temviriyanukul P., Lertmongkolaksorn T., Supasawat P., Pitchakarn P., Thiyajai P., Nusuetrong P., Phochantachinda S., Chansawhang A., Chantong B. Phikud Navakot extract attenuates lipopolysaccharide-induced inflammatory responses through inhibition of ERK1/2 phosphorylation in a coculture system of microglia and neuronal cells. J. Ethnopharmacol. 2022;296:115440. doi: 10.1016/j.jep.2022.115440. [DOI] [PubMed] [Google Scholar]
- 31.Davidson J.M., Wong C.T., Rai-Bhogal R., Li H., Crawford D.A. Prostaglandin E2 elevates calcium in differentiated neuroectodermal stem cells. Mol. Cell. Neurosci. 2016;74:71–77. doi: 10.1016/j.mcn.2016.03.010. [DOI] [PubMed] [Google Scholar]
- 32.Novo M., Freire S., Al-Soufi W. Critical aggregation concentration for the formation of early Amyloid-β (1-42) oligomers. Sci. Rep. 2018;8:1783. doi: 10.1038/s41598-018-19961-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cai Q., Li Y., Pei G. Polysaccharides from Ganoderma lucidum attenuate microglia-mediated neuroinflammation and modulate microglial phagocytosis and behavioural response. J. Neuroinflamm. 2017;14:63. doi: 10.1186/s12974-017-0839-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.He Y., Cui J., Lee J.C., Ding S., Chalimoniuk M., Simonyi A., Sun A.Y., Gu Z., Weisman G.A., Wood W.G., et al. Prolonged exposure of cortical neurons to oligomeric amyloid-β impairs NMDA receptor function via NADPH oxidase-mediated ROS production: Protective effect of green tea (-)-epigallocatechin-3-gallate. ASN Neuro. 2011;3:e00050. doi: 10.1042/AN20100025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chiang M.C., Nicol C.J.B., Cheng Y.C., Yen C., Lin C.H., Chen S.J., Huang R.N. Nanogold Neuroprotection in Human Neural Stem Cells Against Amyloid-beta-induced Mitochondrial Dysfunction. Neuroscience. 2020;435:44–57. doi: 10.1016/j.neuroscience.2020.03.040. [DOI] [PubMed] [Google Scholar]
- 36.Ng N.S., Ooi L. A Simple Microplate Assay for Reactive Oxygen Species Generation and Rapid Cellular Protein Normalization. Bio Protoc. 2021;11:e3877. doi: 10.21769/BioProtoc.3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 38.Chen Z., Zhong C. Oxidative stress in Alzheimer’s disease. Neurosci. Bull. 2014;30:271–281. doi: 10.1007/s12264-013-1423-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cai Z., Hussain M.D., Yan L.J. Microglia, neuroinflammation, and beta-amyloid protein in Alzheimer’s disease. Int. J. Neurosci. 2014;124:307–321. doi: 10.3109/00207454.2013.833510. [DOI] [PubMed] [Google Scholar]
- 40.Wang Q., Xie C. Microglia activation linking amyloid-β drive tau spatial propagation in Alzheimer’s disease. Front. Neurosci. 2022;16:951128. doi: 10.3389/fnins.2022.951128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van Praag H., Schinder A.F., Christie B.R., Toni N., Palmer T.D., Gage F.H. Functional neurogenesis in the adult hippocampus. Nature. 2002;415:1030–1034. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hashiguchi A., San Thawtar M., Duangsodsri T., Kusano M., Watanabe K.N. Biofunctional properties and plant physiology of Kaempferia spp.: Status and trends. J. Funct. Foods. 2022;92:105029. doi: 10.1016/j.jff.2022.105029. [DOI] [Google Scholar]
- 43.Tonsomboon A., Prasanth M.I., Plaingam W., Tencomnao T. Kaempferia parviflora Rhizome Extract Inhibits Glutamate-Induced Toxicity in HT-22 Mouse Hippocampal Neuronal Cells and Extends Longevity in Caenorhabditis elegans. Biology. 2021;10:264. doi: 10.3390/biology10040264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Phachonpai W., Maharun S., Tong-Un T., Muchimapura S., Wattanathorn J. The functional effect of kaempferia parviflora on ischemic stroke in rats. Am. J. Agric. Biol. Sci. 2012;7:173–179. [Google Scholar]
- 45.Youn K., Lee J., Ho C.-T., Jun M. Discovery of polymethoxyflavones from black ginger (Kaempferia parviflora) as potential β-secretase (BACE1) inhibitors. J. Funct. Foods. 2016;20:567–574. doi: 10.1016/j.jff.2015.10.036. [DOI] [Google Scholar]
- 46.Seo S.H., Lee Y.C., Moon H.I. Acetyl-cholinesterase Inhibitory Activity of Methoxyflavones Isolated from Kaempferia parviflora . Nat. Prod. Commun. 2017;12:21–22. doi: 10.1177/1934578X1701200107. [DOI] [PubMed] [Google Scholar]
- 47.Azuma T., Tanaka Y., Kikuzaki H. Phenolic glycosides from Kaempferia parviflora. Phytochemistry. 2008;69:2743–2748. doi: 10.1016/j.phytochem.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 48.Okabe Y., Shimada T., Horikawa T., Kinoshita K., Koyama K., Ichinose K., Aburada M., Takahashi K. Suppression of adipocyte hypertrophy by polymethoxyflavonoids isolated from Kaempferia parviflora. Phytomedicine. 2014;21:800–806. doi: 10.1016/j.phymed.2014.01.014. [DOI] [PubMed] [Google Scholar]
- 49.Horigome S., Yoshida I., Tsuda A., Harada T., Yamaguchi A., Yamazaki K., Inohana S., Isagawa S., Kibune N., Satoyama T., et al. Identification and evaluation of anti-inflammatory compounds from Kaempferia parviflora . Biosci. Biotechnol. Biochem. 2014;78:851–860. doi: 10.1080/09168451.2014.905177. [DOI] [PubMed] [Google Scholar]
- 50.Kongdang P., Jaitham R., Thonghoi S., Kuensaen C., Pradit W., Ongchai S. Ethanolic extract of Kaempferia parviflora interrupts the mechanisms-associated rheumatoid arthritis in SW982 culture model via p38/STAT1 and STAT3 pathways. Phytomedicine. 2019;59:152755. doi: 10.1016/j.phymed.2018.11.015. [DOI] [PubMed] [Google Scholar]
- 51.Sae-Wong C., Matsuda H., Tewtrakul S., Tansakul P., Nakamura S., Nomura Y., Yoshikawa M. Suppressive effects of methoxyflavonoids isolated from Kaempferia parviflora on inducible nitric oxide synthase (iNOS) expression in RAW 264.7 cells. J. Ethnopharmacol. 2011;136:488–495. doi: 10.1016/j.jep.2011.01.013. [DOI] [PubMed] [Google Scholar]
- 52.Toda K., Hitoe S., Takeda S., Shimoda H. Black ginger extract increases physical fitness performance and muscular endurance by improving inflammation and energy metabolism. Heliyon. 2016;2:e00115. doi: 10.1016/j.heliyon.2016.e00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bei D., An G. Pharmacokinetics and tissue distribution of 5,7-dimethoxyflavone in mice following single dose oral administration. J. Pharm. Biomed. Anal. 2016;119:65–70. doi: 10.1016/j.jpba.2015.11.025. [DOI] [PubMed] [Google Scholar]
- 54.Mekjaruskul C., Jay M., Sripanidkulchai B. Pharmacokinetics, bioavailability, tissue distribution, excretion, and metabolite identification of methoxyflavones in Kaempferia parviflora extract in rats. Drug Metab. Dispos. 2012;40:2342–2353. doi: 10.1124/dmd.112.047142. [DOI] [PubMed] [Google Scholar]
- 55.Horigome S., Yoshida I., Ito S., Inohana S., Fushimi K., Nagai T., Yamaguchi A., Fujita K., Satoyama T., Katsuda S.I., et al. Inhibitory effects of Kaempferia parviflora extract on monocyte adhesion and cellular reactive oxygen species production in human umbilical vein endothelial cells. Eur. J. Nutr. 2017;56:949–964. doi: 10.1007/s00394-015-1141-5. [DOI] [PubMed] [Google Scholar]
- 56.Varga B.V., Hádinger N., Gócza E., Dulberg V., Demeter K., Madarász E., Herberth B. Generation of diverse neuronal subtypes in cloned populations of stem-like cells. BMC Dev. Biol. 2008;8:89. doi: 10.1186/1471-213X-8-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kim C., Hwang J.K. The 5,7-Dimethoxyflavone Suppresses Sarcopenia by Regulating Protein Turnover and Mitochondria Biogenesis-Related Pathways. Nutrients. 2020;12:1079. doi: 10.3390/nu12041079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Phung H.M., Lee S., Hong S., Lee S., Jung K., Kang K.S. Protective Effect of Polymethoxyflavones Isolated from Kaempferia parviflora against TNF-α-Induced Human Dermal Fibroblast Damage. Antioxidants. 2021;10:1609. doi: 10.3390/antiox10101609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jakhar R., Paul S., Park Y.R., Han J., Kang S.C. 3,5,7,3′,4′-Pentamethoxyflavone, a quercetin derivative protects DNA from oxidative challenges: Potential mechanism of action. J. Photochem. Photobiol. B Biol. 2014;131:96–103. doi: 10.1016/j.jphotobiol.2014.01.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material.