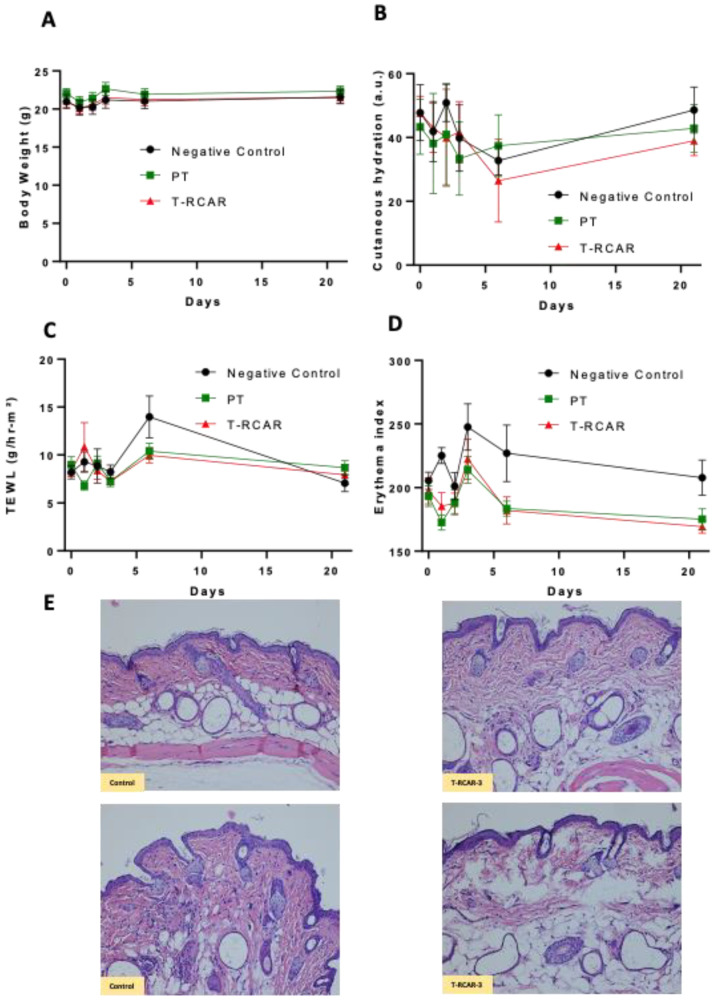
Figure 6.
In vivo dermal toxicity test (repeated dose). (A) Body weight of mice was measured one day before, and on days 1, 2, 3, 6, and 21 after daily T-RCAR gel topical treatment (100 μM R-carvedilol). (B) Cutaneous hydration levels as measured with the corneometer one day before, and days 1, 2, 3, 6, and 21. On day 6, T-RCAR showed reduced hydration in 4 out of 6 mice < 30, indicating skin dryness, but recovered at 3 weeks. (C) Transepidermal water loss (TEWL) for skin barrier function, measured by the Tewameter one day before, and days 1, 2, 3, 6, and 3 weeks after T-RCAR topical treatment was initiated; TEWL < 25: normal condition. (D) Mexameter was used to measure erythema, one day before, and days 1, 2, 3, 6, and 3 weeks after T-RCAR topical treatment was initiated; <330: minimal erythema. Sample size: n = 4 for control; and n = 6 for PT or T-RCAR. Statistical analysis was based on repeated measures ANOVA. For most parameters, there was no significant difference between the three groups, except the erythema index, where the control group showed a slightly increased erythema. (E) Representative H&E-stained images for skin tissues collected from control mice and T-RCAR-3-treated mice.