Fig. 4.
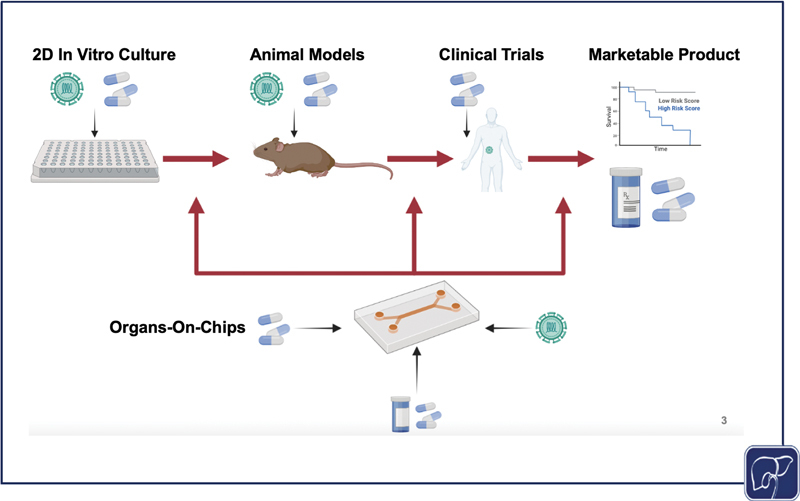
The standard process of disease study and drug development progresses linearly, starting with in vitro testing, moving next to animal models, then conducting clinical trials for drugs and using samples from diseased patients, finally resulting in compounds that are brought to market and clinical data from diseased patients that can be used to predict outcomes and further study the disease. However, a physiomimetic platform allows for a dynamic interplay between in vitro and animal models, and clinical data. If effective, in vitro models can be used to corroborate findings from animal models, predict outcomes from animal models, test and study clinical diseases and treatments in vivo, and predict treatment outcomes for patients as a personalized medicine approach. All of this information can enhance and expedite bench to clinic pipeline (the image was generated using Biorender).
