Abstract
Background: Efferocytosis refers to the physiological clearance process of apoptotic cells by specialized and non-phagocytes and it is essential in human health and disease. However, there is a lack of comprehensive and objective reports on the current status of efferocytosis research. Here, we visually analyzed the hotspots and trending issues of efferocytosis research with bibliometric analysis. Methods: Relevant publications were obtained from the Web of Science Core Collection on February 18, 2022. We performed bibliometric and visual analysis using CiteSpace, VOSviewer, Microsoft Excel 2019, and the online Bibliometric platform. Results: A total of 1007 publications on efferocytosis were retrieved. The number of efferocytosis studies increased rapidly from 2006 to 2021. The country that published the most efferocytosis related articles was the USA and the most productive institutions were Harvard University and Brigham and Women’s Hospital. The most prolific and influential author was I. Tabas of Columbia University. Frontiers in Immunology published the most research papers on efferocytosis, the while Journal of Immunology had the highest co-citation frequency. The high-frequency keywords were “efferocytosis”, “inflammation”, “apoptotic cells”, “macrophages”, and “apoptosis”. The analysis of keywords with the strongest citation bursts identified “cell” and “resolution” as emerging hotspots. Conclusion: Our results demonstrated that efferocytosis research increased steadily within the past decade. Current efferocytosis studies focus on three main aspects: mechanisms, basic biology, and potential role in disease. The research trends included the cellular players of the efferocytosis process and the role of efferocytosis in inflammation resolution. This bibliometric analysis presented a comprehensive overview of efferocytosis research and provided valuable references and ideas for scholars interested in this field.
Keywords: Efferocytosis, apoptotic cell clearance, inflammation, bibliometric analysis, visual analysis
Introduction
Approximately 0.4% of an adult’s estimated 37.2 trillion cells die daily, which is essential for normal metabolism in the human body [1,2]. The effective removal of dying cells and cellular debris is a key factor in maintaining tissue homeostasis and the normal function of an organism [3]. Both apoptotic and non-apoptotic dying cells display and release molecular cues, such as “find-me” and “eat-me” signals, to recruit phagocytes and direct the recognition of apoptotic or dying cells and the subsequent phagocytic and immune response [4,5]. This multi-step cell clearance process is termed “efferocytosis”, which is the Latin for “to take to the grave” [6]. In cell biology, efferocytosis refers to apoptotic cell engulfment and decomposition by both specialized and non-specialized phagocytes [7].
Efferocytosis is critical for resolving pathological events, such as infection, tissue damage, and inflammation [8]. Defective efferocytosis can cause apoptotic cell accumulation in the inflammatory microenvironment, subsequently leading to cytolysis, cellular necrosis, and the generation of proinflammatory cell content [9]. Failed or impaired clearance of apoptotic cells is strongly associated with many chronic inflammatory diseases [10]. For example, efferocytosis has been identified as a key contributing factor in atherosclerotic lesion development [11]. Improper apoptotic cell clearance is an essential cause of persistent lung inflammation. Several chronic pulmonary diseases, including asthma, chronic obstructive pulmonary disease, and cystic fibrosis are characterized by abnormal apoptotic cell accumulation in the lung [12]. Moreover, defects in efferocytosis can induce self-antigen break of tolerance and trigger autoimmunity, resulting in the development of autoimmune diseases [13]. Recently, many researchers have considered efferocytosis a promising new target for probing the causes and therapies of neurodegenerative disease, diabetes, and cancer [14]. Therefore, there remains much to learn about the physiological and pathological functions of efferocytosis.
Bibliometrics presents a method for quantitatively and statistically visualizing evidence based on information in published studies in a given research field, which includes the cooperation and contribution of countries, institutions, authors, and journals [15]. Bibliometric analysis can also aid in understanding the knowledge structure and evaluate emerging trends in the scientific research of a particular area [16], which are advantages not available with other methods such as traditional reviews, meta-analyses, and evidence mapping. Bibliometrics has become increasingly recognized as a valuable method for developing guidelines and exploring research trends [17]. The most commonly used bibliometric tools for visualizing bibliometric information are CiteSpace [18] and VOSviewer [19], which have been broadly applied in medicine [17], biology [16], and immunology [20].
To our knowledge, there has been no bibliometric analysis on efferocytosis to date. Consequent to this knowledge gap, we aimed to map the full picture of efferocytosis research and determine trending research questions. Moreover, this bibliometric analysis was aimed at providing researchers with a macro-perspective of this field and lay the foundation for future research.
Materials and methods
Data collection
Data were retrieved from the Science Citation Index-Expanded (SCI-E) and Social Sciences Citation Index (SSCI) of the Web of Science Core Collection (WoSCC) on 18 February, 2022. The search phrase was: Topics = (efferocytosis) OR (efferocytotic) and the publication years were limited to 2006-2021. The publication language was restricted to English. Original research articles and reviews were the only publication types available. Figure 1 depicts a comprehensive overview of the search strategy. The retrieved documents were exported in the form of “Full Record and Cited References” and “Plain Text”. Two researchers checked the data collection and entry independently, where any differences between their results were resolved by consensus through discussion or consulting experts in the field.
Figure 1.

Flow diagram of the literature selection process in this study.
Data analysis
VOSviewer 1.6.16, CiteSpace 5.8.R3, Microsoft Excel 2019, and the Bibliometric platform (https://bibliometric.com) were used for the bibliometric and visual analysis.
CiteSpace is a bibliometric software that specializes in analyzing distributions, key points, knowledge structures, future trends, and dynamics in a scientific field [18]. Here, CiteSpace was used to analyze the co-occurrences of institutions, keyword bursts, citation bursts, co-citation relationship of authors and references, reference timeline, and journal dual-maps. In CiteSpace visualization, nodes refer to references, authors, and institutions. The node size reflects the co-occurrence frequencies of the items, the color rings represent different years, and the lines between nodes indicate the co-occurrence relationships of items.
VOSviewer is a scientometrics analysis tool that enables the creation and visualization of knowledge structures and depicts three visualization map types: cluster, overlay, and density color [19]. Unlike the commonly used bibliometric tools, VOSviewer emphasizes bibliometric graphical representation, which is particularly useful for visualizing large-scale data intuitively. We used VOSviewer to visualize networks, including the co-occurrence of keywords and co-cited authors.
The collaboration and publication analysis of countries was conducted using the Bibliometric platform. The efferocytosis publication trends were analyzed with Microsoft Excel 2019.
Results
Annual growth trend
A total of 1007 eligible papers on efferocytosis research were published between 2006 and 2021. Despite fluctuating declines at certain time points, there was an overall upward trend in the number of efferocytosis-related publications (Figure 2A). Notably, research activity peaked in 2017-2021, where 636 papers were published during this 5-year period, which accounted for >60% of the total number of related publications.
Figure 2.

A. The number of publications annually related to efferocytosis (2006-2021). B. Top 10 productive countries in the field of efferocytosis (2006-2021).
Distributions by countries/regions and institutions
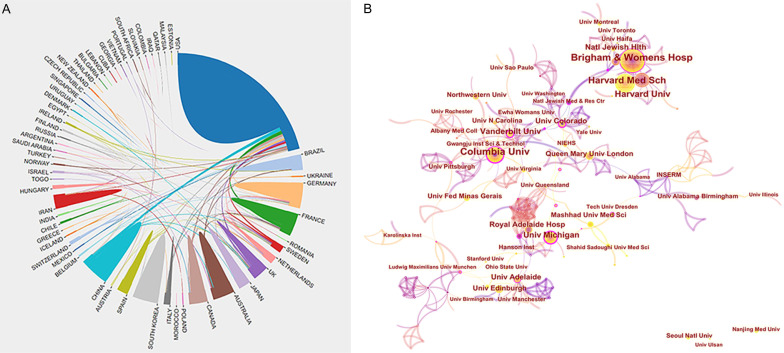
A total of 1007 papers were published by 69 countries/regions and 1243 institutions. The US published the most articles (n = 481), followed by China (n = 102), Germany (n = 86), the UK (n = 84), and France (n = 71) (Figure 2B and Table 1). The US published >45% of efferocytosis research worldwide, indicating that this country is a leader in this field.
Table 1.
The top 10 countries/regions involved in efferocytosis research
| Rank | Article counts | Centrality score | Country |
|---|---|---|---|
| 1 | 481 | 0.10 | USA |
| 2 | 102 | 0.00 | China |
| 3 | 86 | 0.50 | Germany |
| 4 | 84 | 0.59 | UK |
| 5 | 71 | 0.19 | France |
| 6 | 65 | 0.00 | South Korea |
| 7 | 61 | 0.10 | Canada |
| 8 | 51 | 0.35 | Australia |
| 9 | 44 | 0.32 | Japan |
| 10 | 43 | 0.23 | Brazil |
The centrality score evaluates the significance of nodes in a network [21]. More frequent cooperation is associated with greater centrality in a collaborative network. Figure 3A depicts the cooperation networks across countries/regions, where the UK had the highest centrality score (0.59), followed by Germany (0.50), Australia (0.35), Japan (0.32), and Brazil (0.23).
Figure 3.
A. Cooperation network of prolific countries/regions. B. Visualization map of institutions’ cooperative relations.
The distribution of institutions contributing to publications in the field was analyzed with CiteSpace. The five most prolific institutions (papers published) were as follows (Figure 3B and Table 2): Harvard University (n = 85), Brigham and Women’s Hospital (n = 67), Harvard Medical School (n = 64), Institut national de la santé et de la recherche médicale (n = 58), and Columbia University (n = 48). The top 10 institutions were from the USA (n = 8), France (n = 1), and the UK (n = 1). Ranked according to centrality, the top three institutions were the University of Colorado System (0.26), Columbia University (0.22), and University of Michigan (0.21).
Table 2.
The top 10 institutions involved in efferocytosis research
| Rank | Article counts | Institution | Country | Centrality score |
|---|---|---|---|---|
| 1 | 85 | Harvard University | USA | 0.04 |
| 2 | 67 | Brigham and Women’s Hospital | USA | 0.17 |
| 3 | 64 | Harvard Medical School | USA | 0.01 |
| 4 | 58 | Institut National de la Sante et de la Recherche Medicale | France | 0.11 |
| 5 | 48 | Columbia University | USA | 0.22 |
| 6 | 35 | University of London | UK | 0.00 |
| 7 | 32 | National Institutes of Health | USA | 0.03 |
| 8 | 30 | National Jewish Health | USA | 0.01 |
| 9 | 30 | University of Colorado System | USA | 0.26 |
| 10 | 27 | University of Michigan | USA | 0.21 |
Authors and co-cited authors
In total, 5218 authors contributed to efferocytosis research. Table 3 lists the top 10 most prolific authors. Based on publication counts, I. Tabas of Columbia University was the most productive author (n = 37), followed by C.N. Serhan of Brigham and Women’s Hospital (n = 36), J. Dalli of Queen Mary University of London (n = 28), S. Hodge of Royal Adelaide Hospital (n = 22), and P.M. Henson of University of Colorado (n = 17). I. Tabas was ranked first again based on citations in this field (n = 3572). Assessment of the authors’ influence based on citations and the H-index again revealed that I. Tabas was the most influential scholar in efferocytosis research. Figure 4 depicts the nine colors representing the nine author clusters. Typically, there were close collaborations within the same cluster, such as between I. Tabas and G. Fredman and between C.N. Serhan and J. Dalli. Furthermore, there was active cooperation among the clusters, such as between I. Tabas and E.B. Thorp, E. Abraham and W. Janssen, and C.N. Serhan and A. Ariel.
Table 3.
The top 10 authors of efferocytosis research
| Rank | Author | Articles Counts | Centrality | Total Citations | Average Citations | H-index |
|---|---|---|---|---|---|---|
| 1 | Tabas I | 37 | 0.03 | 3572 | 96.54 | 31 |
| 2 | Serhan CN | 36 | 0.01 | 2982 | 82.83 | 29 |
| 3 | Dalli J | 28 | 0.00 | 2276 | 81.29 | 25 |
| 4 | Hodge S | 22 | 0.00 | 969 | 44.05 | 16 |
| 5 | Henson PM | 17 | 0.01 | 1775 | 104.41 | 17 |
| 6 | Perretti M | 16 | 0.00 | 926 | 57.88 | 11 |
| 7 | Birge RB | 16 | 0.00 | 739 | 46.19 | 13 |
| 8 | Teixeira MM | 16 | 0.00 | 324 | 20.25 | 10 |
| 9 | Abraham E | 15 | 0.00 | 700 | 46.67 | 12 |
| 10 | Mccauley LK | 15 | 0.00 | 579 | 38.60 | 10 |
Figure 4.
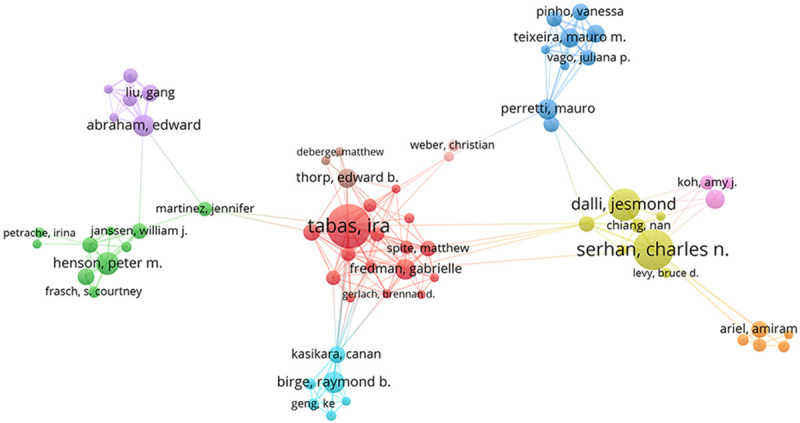
Inter-author collaborative network map.
Analysis of journals
The analysis determined that 376 academic journals published 1007 efferocytosis-related papers. Table 4 presents the characteristics of the top 10 most productive journals, which published a total of 266 articles, constituting 26.4% of total publications. Frontiers in Immunology published the most articles (n = 72), followed by Journal of Immunology (n = 39) and PLOS ONE (n = 33). Five of the top 10 journals were in the first quartile (Q1) and eight had an impact factor (IF) >5. The journal co-citation numbers can be measured to determine their impact on a particular research area. Journal of Immunology (n = 1863), Frontiers in Immunology (n = 1643), and Arteriosclerosis Thrombosis and Vascular Biology (n = 1257) contained the most citations (Table 4).
Table 4.
The top 10 journals of efferocytosis research
| Rank | Journal | Article counts | Country | Journal citation reports (2021) | Impact factors (2021) | Total number of citations | Mean number of citations | H-index |
|---|---|---|---|---|---|---|---|---|
| 1 | Frontiers in Immunology | 72 | Switzerland | Q1 | 8.786 | 1643 | 22.82 | 23 |
| 2 | Journal of Immunology | 39 | USA | Q2 | 5.426 | 1863 | 47.77 | 23 |
| 3 | PLOS ONE | 33 | USA | Q2 | 3.752 | 1220 | 36.79 | 17 |
| 4 | Arteriosclerosis Thrombosis and Vascular Biology | 22 | USA | Q1 | 10.514 | 1257 | 57.14 | 17 |
| 5 | Journal of Leukocyte Biology | 20 | USA | Q2 | 6.011 | 713 | 35.65 | 13 |
| 6 | Cell Death & Disease | 18 | UK | Q1 | 9.685 | 396 | 22.00 | 11 |
| 7 | Cells | 16 | Switzerland | Q2 | 7.666 | 48 | 3.00 | 4 |
| 8 | Scientific Reports | 16 | UK | Q2 | 4.996 | 288 | 18.00 | 11 |
| 9 | Circulation Research | 15 | USA | Q1 | 23.213 | 1036 | 69.07 | 14 |
| 10 | FASEB Journal | 15 | USA | Q1 | 5.834 | 795 | 53.00 | 11 |
The dual-map journal overlay represents the topic distribution of relationships between academic journals. The left and right sides of the map depict the citing and cited journals, respectively. There was only one primary citation path (Figure 5). The orange route indicated that the studies published in molecular/biology/genetic journals were generally cited by molecular/biology/immunology journals.
Figure 5.
A dual-map overlay analysis of journals.
Keyword analysis
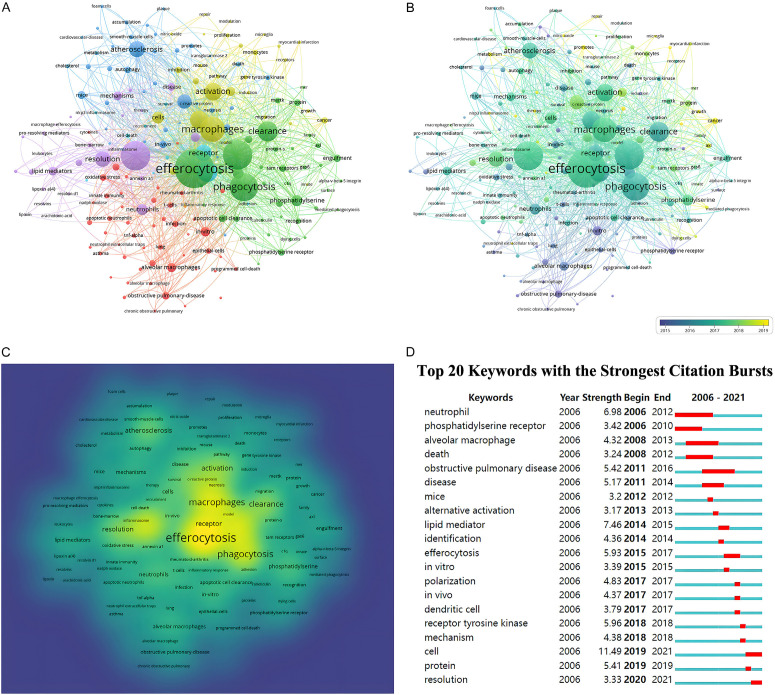
As keywords summarize research topics, keyword analysis yields insight into the hotspots of a particular research area. Here, a total of 4115 keywords were retrieved, of which 194 occurred >10 times. Figure 6A depicts the keywords with the highest frequency. “Efferocytosis” was the most frequent keyword (429 co-occurrences), followed by “inflammation” (291 co-occurrences) and “apoptotic cells” (288 co-occurrences). Cluster analysis can be used to determine the knowledge domain of a research field. Here, five clusters were identified based on the link strength of keyword co-occurrence (Figure 6A). Cluster 1 (green) was composed of “efferocytosis”, “phagocytosis”, “clearance”, and “engulfment”. Cluster 2 (red) focused on lung disease and included “alveolar macrophage”, “obstructive pulmonary disease”, “lung”, “epithelial cells”, and “asthma”. Cluster 3 (blue) was primarily related to cardiovascular disease and consisted of “atherosclerosis”, “cholesterol”, “plaque”, “smooth muscle cells”, “foam cells”, and “metabolism”. The area color depth demonstrated that the keywords were distributed in chronological order (Figure 6B). Before 2015, most efferocytosis research concentrated on lung disease, while the most recently identified research hotspots indicated “cancer”, “metabolism”, and “therapy” as emerging fields. We visualized the keyword frequencies in a density map (Figure 6C). Keywords with citation bursts were defined as those cited frequently during a given time period. “Cell” had the highest burst strength (11.49), followed by “lipid mediator” (7.46) and “neutrophil” (6.98) (Figure 6D). It should be noted that as of 2021, “cell” and “resolution” were in bursts.
Figure 6.
Keywords analysis in publications related to efferocytosis (2006-2021). A. Clustering co-occurrence map of keywords. B. Distribution of keywords based on the average time of appearance. C. Density network visualization graph of keywords. D. Top 20 keywords with the strongest citation bursts.
Analysis of co-cited references and reference bursts
Table 5 presents the top 10 cited articles. Among them, nine papers were cited >300 times. The most cited reference was published by Epelman et al. [22] in Immunity (n = 728), followed by those of Ortega-Gomez et al. [23] in EMBO Molecular Medicine (n = 416), and Liao et al. [24] in Cell Metabolism (n = 397). References with citation bursts are frequently cited over a certain period. Figure 7 depicts the top 20 references with the strongest citation bursts. The publication with the strongest bursts (28.43) (“Apoptotic cell clearance: basic biology and therapeutic potential”) was published in Nature Reviews Immunology by Poon et al. in 2014 [25]. In particular, two references remained in burst until 2021.
Table 5.
Top 10 cited references for efferocytosis research
| Rank | Title | Author | Year | Journal | Citation frequency |
|---|---|---|---|---|---|
| 1 | Embryonic and Adult-Derived Resident Cardiac Macrophages Are Maintained through Distinct Mechanisms at Steady State and during Inflammation | Epelman S | 2014 | Immunity | 738 |
| 2 | Resolution of inflammation: an integrated view | Ortega-Gomez A | 2013 | EMBO Molecular Medicine | 416 |
| 3 | Macrophage Autophagy Plays a Protective Role in Advanced Atherosclerosis | Liao XH | 2012 | Cell Metabolism | 397 |
| 4 | Macrophage defense mechanisms against intracellular bacteria | Weiss G | 2015 | Immunological Reviews | 396 |
| 5 | Macrophage Dysfunction Impairs Resolution of Inflammation in the Wounds of Diabetic Mice | Khanna S | 2010 | PLOS ONE | 360 |
| 6 | Inflammation and its resolution in atherosclerosis: mediators and therapeutic opportunities | Back M | 2019 | Nature Reviews Cardiology | 325 |
| 7 | Specific lipid mediator signatures of human phagocytes: microparticles stimulate macrophage efferocytosis and pro-resolving mediators | Dalli J | 2012 | Blood | 316 |
| 8 | Apoptosis and Clearance of Apoptotic Cells | Nagata S | 2018 | Annual Review of Immunology | 311 |
| 9 | Burying the dead - The impact of failed apoptotic cell removal (efferocytosis) on chronic inflammatory lung disease | Vandivier RW | 2006 | Chest | 306 |
| 10 | Neutrophils orchestrate post-myocardial infarction healing by polarizing macrophages towards a reparative phenotype | Horckmans M | 2017 | European Heart Journal | 297 |
Figure 7.
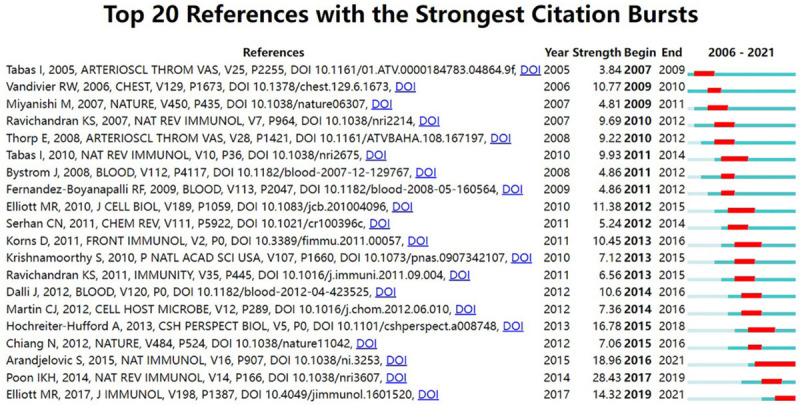
Top 20 references with the strongest citation bursts (2006-2021).
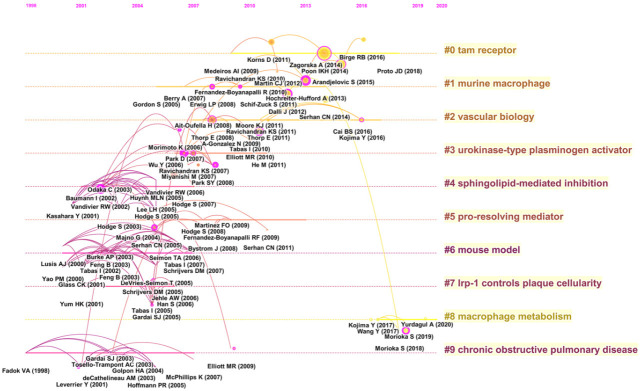
Analysis of cited references is considered a key part of bibliometric research. Here, the co-citation correlation analysis of 43,706 cited references from 1007 articles led to the creation of a cluster network map. The visualized network of co-cited articles contained 1026 nodes and 3007 links (Figure 8A), where each node represented a cited reference. The links between nodes indicated the frequency of the same article being cited. The node diameter is proportional to the frequency with which the cited reference is quoted. Figure 8B illustrates the largest 10 clusters of the reference co-citation network. The largest cluster was “TAM receptor” (#0), followed by “murine macrophage” (#1) and “vascular biology” (#2). The 10 clusters are presented as a timeline view (Figure 9) that depicts the clusters together with horizontal timelines in descending order, thereby demonstrating the interaction and evolution of the references. We determined that the recently cited references on efferocytosis focused on macrophage metabolism.
Figure 8.
Analysis of co-cited references. A. The network of co-cited references. B. Clustering visualization map of the co-cited references.
Figure 9.
Timeline visualization map of the co-cited references.
Discussion
General information
According to the WoSCC database, 5218 authors from 1243 institutions in 69 countries/regions published 1007 papers in 376 academic journals from 2006 to 2021. The overall upward trend in the production of efferocytosis publications indicated that efferocytosis is becoming a popular research field. In fact, the earliest efferocytosis research we retrieved from WoSCC was published in 2006. Since then, efferocytosis research has grown steadily. Notably, the number of publications on efferocytosis accounted for >60% of the total production in 2017-2021, which indicated that the field has entered a rapid development phase.
Our analysis of contributions according to country revealed that the USA and China dominate this field. The USA was the most productive country, contributing >40% of all publications. This may be associated with the economic development and financial investment in scientific research of these top contributing countries. Despite being ranked among the top 10 countries, the USA (0.10) and China (0.00) did not have the highest centrality scores. Rather, the UK (0.59) ranked highest in centrality scores, followed by Germany (0.50), indicating that they had a crucial role in global collaboration on efferocytosis research. Of the top 10 institutions, eight were in the USA and one each was in France and the UK. Harvard University published the most papers. Additionally, network density revealed that there was active collaboration between institutions.
Identifying the contributions of leading authors in a particular field can assist scholars in progressing along the research path and provide guidance [15]. Our analysis determined that I. Tabas of Columbia University authored the most publications and had the most citations, indicating his distinguished contribution to efferocytosis research. The publication and citation numbers revealed that C.N. Serhan of Brigham and Women’s Hospital, J. Dalli of Queen Mary University of London, S. Hodge of Royal Adelaide Hospital, and P.M. Henson of University of Colorado are also leaders in this field. Therefore, these researchers and their teams are more likely to publish significant papers related to efferocytosis in the future. Accordingly, these top researchers might be suitable candidates for academic exchange and cooperation.
The journal analysis revealed that Frontiers in Immunology had the highest number of efferocytosis articles while Journal of Immunology was the most cited journal. Overall, the top 10 journals on efferocytosis research were primarily in the fields of immunology, cell biology, and multidisciplinary sciences. This was in agreement with the dual-map overlay analysis, which demonstrated that the primary citation pathway in efferocytosis research was associated with molecular, biology, and immunology. Our results also reflect the fact that current efferocytosis-related research mainly focuses on basic research, while translational medical studies remain scarce.
References with citation bursts could somewhat reflect hotspots and dynamics in efferocytosis-related research [20]. The strongest citation burst was from a review by Poon et al. [25] (28.43, 2017-2019), which provided a comprehensive overview of the underlying biology and curative potential of apoptotic cell clearance. It is worth noting that the burst of two publications is ongoing: Elliott et al. [9] comprehensively reviewed efferocytosis signaling in inflammation regulation in 2017 and Arandjelovic and Ravichandran [26] highlighted the role of apoptotic cell clearance in tissue homeostasis in Nature Immunology in 2015. The collection of co-cited references can partially represent the knowledge base [21]. The cluster analysis of the reference co-citation network revealed that efferocytosis studies mainly focused on mechanisms, basic biology, and related diseases.
Hotspots and frontiers
As information explosions continue, keeping pace with the most recent research findings is becoming increasingly challenging [16]. Bibliometrics is a useful tool for evaluating the features of literature in a specific field over a specific timeframe and yields a wealth of valuable data [17]. In the present bibliometric analysis, we objectively identified efferocytosis research hotspots and emerging trends by analyzing keyword bursts, keyword co-occurrence, keyword overlay, reference bursts, and the reference timeline and summarized the following three aspects described below.
Molecular mechanisms of efferocytosis
The initiation and enactment of efferocytosis guided by various signaling molecules (mediators from apoptotic cells, bridging molecules, and phagocyte receptors) requires the accurate recognition and removal of apoptotic cells [27]. Typically, three major stages characterize efferocytosis: recruitment, recognition, and engulfment/processing [28]. Recruitment involves the communication of dying cells with nearby phagocytes. Apoptotic particles release “find-me” signals that recruit motile phagocytes at the onset of apoptosis [29]. Recognition refers to phagocyte connection and interaction with apoptotic cells. Here, phagocytes recognize the binding ligands of apoptotic cell membranes, also referred to as “eat-me” signals, through specific surface receptors (“eat-me” markers). These interactions activate Ras-related C3 botulinum toxin substrate 1 (Rac1)-mediated signaling pathways in phagocytes, which lead to cytoskeletal rearrangements, thereby promoting their engulfment capacity. In turn, this facilitates engulfment/processing, which involves apoptotic body internalization that enables macrophages to process them through the phagolysosome.
It is worth mentioning that our analysis of co-cited references revealed two important groups of efferocytosis-associated molecules: “TAM receptors” and “pro-resolving mediators”. Principally expressed on phagocytes, TAM receptors are a receptor tyrosine kinase family that includes TYRO3 protein tyrosine kinase 3 (Tyro3), AXL receptor tyrosine kinase (Axl), and c-Mer proto-oncogene tyrosine kinase (MerTK), which function via the bridging molecules of apoptotic cells [30]. For example, TAM receptors can bind to phosphatidylserine, the most studied eat-me signal, on apoptotic cells via bridging molecules that include growth arrest-specific 6 (Gas6) and protein S (Pros1) [31]. The ligand binding stimulates TAM receptor dimerization and phosphorylation, which ultimately results in activation of the rhodopsin (Rho) family of small GTPases. This programmed cascade of signaling events induces cytoskeleton phagocyte rearrangement and the phagocytosis of apoptotic cells. The efficient clearance of apoptotic cells requires the inhibition of proinflammatory cytokines and the production of anti-inflammatory cytokines [32]. Specialized pro-resolving mediators (SPMs) derived from essential polyunsaturated fatty acids are autacoid mediators that share a defining action in efferocytosis-mediated inflammation resolution [33]. Among the SPM superfamily are resolvins, lipoxins, maresins, and protectins. The SPM family members all limit further neutrophil infiltration and promote macrophage clearance of apoptotic cells and cellular debris to maintain tissue homeostasis [34].
Cellular players of efferocytosis
Efferocytosis is performed mainly by macrophages and to a lesser extent by specialized and non-specialized phagocytes [4]. The specialized phagocytes include tissue-resident macrophages, monocytes, and immature dendritic cells, which can successively ingest and process various cell corpses at a relatively quick ingestion rate [5]. In contrast, non-specailzed phagocytes (e.g., epithelial cells, fibroblasts, endothelial cells, and other stromal cells) exhibit slower phagocytosis kinetics and are less able to ingest several cell carcasses at once [7]. In the central nervous system, microglia are the main cells with efferocytosis functions [35]. Furthermore, neuronal progenitor cells and astrocytes participate in clearing dead cells and other waste [35]. Overall, efferocytosis is accomplished primarily by macrophages, and to a lesser extent, other types of cells. Notably, new evidence suggested that cellular metabolism modifications are crucial in shaping efferocytic macrophage function and phenotype [36]. Furthermore, our results likewise demonstrate that macrophage metabolism is an emerging research trend in this field. For example, it was recently demonstrated that enhanced glycolysis promoted macrophage efferocytosis and the establishment of an anti-inflammatory milieu induced by efferocytic macrophages [31]. Altered lipid metabolism in macrophages activates transcription factors, such as retinoid X receptor (RXR) and liver X receptor (LXR), thereby promoting the pro-resolving phenotype [37]. Additionally, cholesterol metabolism in macrophages cross-talks with nuclear receptor signaling in efferocytosis [38]. It appears that mitochondrial metabolism is also important for modulating efferocytosis, which is supported by several lines of evidence. Metabolomic analysis of efferocytic macrophages revealed sirtuin 1 signaling activation and enhanced mitochondrial beta-oxidation, which contributed to the upregulation of pro-resolving interleukin (IL)-10 [39]. One main outcome of efferocytosis is the promotion of self-tolerance. Macrophages can crosstalk with regulatory T (Treg) cells, which are potent immune system modulators [40]. By releasing IL-10 and transforming growth factor (TGF)-β, macrophages interact with Treg cells, thereby promoting tolerance and restricting excessive inflammation. In summary, efferocytosis is a strictly regulated process that involves complex interactions between phagocytes and dead or dying cells.
The roles of efferocytosis in inflammation and diseases
Efferocytosis acts as a waste processing mechanism (apoptotic cell clearance) and promotes inflammatory response termination [41]. Currently, it is generally accepted that efferocytosis is a prerequisite for inflammation resolution. Briefly, efferocytosis allows macrophages to acquire a pro-resolving phenotype by reducing proinflammatory cytokine expression (e.g., IL-1, IL-12, and TNF-α) and increasing anti-inflammatory cytokine levels (e.g., TGF-β, IL-10, and SPMs) [9]. SPM synthesis promotes efferocytosis, thereby contributing to inflammation resolution. The most widely known example is efferocytosis promotion of neutrophil inflammation regression. Neutrophils recruited to the inflammation site are central to the development of infection-associated inflammation or sterile inflammation [42]. Upon engulfing apoptotic neutrophils, macrophages undergo phenotypic transition from a proinflammatory phenotype to an anti-inflammatory phenotype. In vitro experiments revealed that macrophages cultured with neutrophil microparticles or apoptotic neutrophils exhibited altered lipid metabolism from proinflammatory prostaglandins and leukotrienes to pro-resolving autacoids [8]. Given the anti-inflammatory properties of efferocytosis, novel therapies targeted at excessive inflammation can be developed based on a better understanding of the molecular players in efferocytosis.
Impaired efferocytosis is associated with human disease. We have presented a few examples in this study, with emphasis on lung disease and cardiovascular disease. The lung is particularly vulnerable to efferocytosis defects as it contains an enormous number and variety of phagocytes, including professional phagocytes (alveolar macrophages and dendritic cells) and non-professional phagocytes (airway epithelial cells) [12]. In 2001, Sexton et al. [43] first discovered that human alveolar epithelial cells can absorb apoptotic eosinophils in asthma, which sparked efferocytosis studies in lung inflammatory disorders. Subsequently, it was discovered that the lungs of people with chronic inflammatory lung diseases, such as asthma, cystic fibrosis (CF), and chronic obstructive pulmonary disease (COPD), contained more apoptotic cells [12]. Alveolar macrophages from CF patients with elastase-mediated degradation of recognition receptors demonstrate impaired efferocytosis and higher apoptotic cell production in the sputum [44]. In humans, COPD exacerbation intensity and frequency were linked to increased eosinophils and reduced eosinophil clearance by macrophages [45]. The fact that apoptotic cells are more prevalent in asthma and that patients with glucocorticoid-resistant disorders have monocytes and alveolar macrophages with proinflammatory skew suggested that impaired efferocytosis may play a role in asthma etiology [46]. Compared to healthy participants, macrophages from patients with asthma exhibited a lower capacity for efferocytosis [12].
Atherosclerosis is the most intensively studied of all cardiovascular diseases associated with defective efferocytosis. It has long been recognized that atherogenesis and plaque stability are related to apoptotic and necrotic debris accumulation [14,47]. Atherosclerotic plaques are formed when modified lipoproteins accumulate within the arterial wall, thereby creating the inflammatory response that leads to leukocyte infiltration into the vessels [48]. While many apoptotic leukocytes occur early in lesion development, they are efficiently cleared from the body. However, efferocytosis begins to fail in advanced plaques. The unremoved apoptotic and necrotic cells undergo secondary necrosis within atherosclerotic plaques, which drive necrotic core formation, plaque enlargement, and plaque rupture [47]. Within early atherosclerotic lesions, effective macrophage efferocytosis appears to alleviate atherosclerosis [49]. Efferocytosis prevents the development of necrotic cores, plaque vulnerability, and acute luminal thrombosis [11]. It is now widely believed that defective efferocytosis is responsible for the formation of necrotic cores in advanced atheroma, which contribute to the formation of unstable plaques. Emerging evidence indicated that the defective efferocytosis in atherosclerosis may be caused by impaired “eat-me” and “don’t eat me” signaling [50].
Defective efferocytosis can result in apoptotic cells rupture, which releases harmful intracellular contents that can trigger autoimmune responses [13]. There is growing evidence that defective efferocytosis is strongly associated with the emergence and development of many autoimmune diseases, such as systemic lupus erythematous, rheumatoid arthritis, and multiple sclerosis [51]. Improper efferocytosis is also involved in other diseases of various systems, such as liver diseases [52], neurodegenerative diseases [35], and diabetes [53]. Recently, several molecules and pathways involved in efferocytosis were considered promising therapeutic targets against cancer [54].
To summarize, studies on the cellular and molecular mechanisms underlying efferocytosis remain in the early phase of understanding but may form the basis for the translational treatment of several human diseases.
Strengths and limitations
To the best of our knowledge, this is the first comprehensive, objective, and visual analysis of publications on efferocytosis. New academic researchers may benefit from our research by gaining a general understanding of the current status, hotspots, evolution, and trends of efferocytosis research with relative ease. Moreover, our results provide a reference for researchers and funding agencies to explore collaboration opportunities and guide investment decisions.
Inevitably, this study has several limitations inherent in bibliometrics. First, data were obtained only from the WoSCC database, which resulted in the inclusion of a non-exhaustive list of publications on efferocytosis. Nevertheless, the WoSCC remains the most commonly used database for bibliometric analysis. Second, as we included only English papers in this analysis, critical studies in other languages may have been overlooked. Third, the WoSCC data are continuously updated and our search results differed slightly from the actual amount of literature available. Nevertheless, we believe that this analysis covered almost all publications from 2006 and the conclusions would not change even if a small amount of new data were to emerge.
Conclusion
In light of the current global trends, efferocytosis-related research is in the phase of rapid development. Publications are mainly in the molecular, biology, and immunology fields. The USA dominates in efferocytosis research. The three main aspects of efferocytosis research were further mechanisms, basic biology, and potential roles in disease. Inflammation resolution and cellular mechanisms might be emerging and promising areas in efferocytosis research.
Compared to traditional reviews, this work presented an objective and systematic perspective on efferocytosis. We believe that our results would be a valuable reference for further research on efferocytosis.
Acknowledgements
This research was supported by the National Natural Science Foundation of China (No. 82071017 and No. 82271134), National Natural Science Foundation of Hubei Province (No. 2021CFB125) and the Fundamental Research Funds for the Central Universities (No. 2042021kf0093).
Disclosure of conflict of interest
None.
References
- 1.Yin C, Heit B. Cellular responses to the efferocytosis of apoptotic cells. Front Immunol. 2021;12:631714. doi: 10.3389/fimmu.2021.631714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hotchkiss RS, Strasser A, McDunn JE, Swanson PE. Cell death. N Engl J Med. 2009;361:1570–1583. doi: 10.1056/NEJMra0901217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Muñoz LE, Leppkes M, Fuchs TA, Hoffmann M, Herrmann M. Missing in action-the meaning of cell death in tissue damage and inflammation. Immunol Rev. 2017;280:26–40. doi: 10.1111/imr.12569. [DOI] [PubMed] [Google Scholar]
- 4.Henson PM. Cell removal: efferocytosis. Annu Rev Cell Dev Biol. 2017;33:127–144. doi: 10.1146/annurev-cellbio-111315-125315. [DOI] [PubMed] [Google Scholar]
- 5.Boada-Romero E, Martinez J, Heckmann BL, Green DR. The clearance of dead cells by efferocytosis. Nat Rev Mol Cell Biol. 2020;21:398–414. doi: 10.1038/s41580-020-0232-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Henson PM, Vandivier RW, Douglas IS. Cell death, remodeling, and repair in chronic obstructive pulmonary disease? Proc Am Thorac Soc. 2006;3:713–717. doi: 10.1513/pats.200605-104SF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gheibi Hayat SM, Bianconi V, Pirro M, Sahebkar A. Efferocytosis: molecular mechanisms and pathophysiological perspectives. Immunol Cell Biol. 2019;97:124–133. doi: 10.1111/imcb.12206. [DOI] [PubMed] [Google Scholar]
- 8.Ge Y, Huang M, Yao YM. Efferocytosis and its role in inflammatory disorders. Front Cell Dev Biol. 2022;10:839248. doi: 10.3389/fcell.2022.839248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elliott MR, Koster KM, Murphy PS. Efferocytosis signaling in the regulation of macrophage inflammatory responses. J Immunol. 2017;198:1387–1394. doi: 10.4049/jimmunol.1601520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Szondy Z, Garabuczi E, Joós G, Tsay GJ, Sarang Z. Impaired clearance of apoptotic cells in chronic inflammatory diseases: therapeutic implications. Front Immunol. 2014;5:354. doi: 10.3389/fimmu.2014.00354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang L, Li H, Tang Y, Yao P. Potential mechanisms and effects of efferocytosis in atherosclerosis. Front Endocrinol [Lausanne] 2021;11:585285. doi: 10.3389/fendo.2020.585285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McCubbrey AL, Curtis JL. Efferocytosis and lung disease. Chest. 2013;143:1750–1757. doi: 10.1378/chest.12-2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kawano M, Nagata S. Efferocytosis and autoimmune disease. Int Immunol. 2018;30:551–558. doi: 10.1093/intimm/dxy055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doran AC, Yurdagul A Jr, Tabas I. Efferocytosis in health and disease. Nat Rev Immunol. 2020;20:254–267. doi: 10.1038/s41577-019-0240-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang S, Wu K, Zhang Z, Xu Z, Wu J, Xu S. Mapping theme trends and recognizing research hot spots in the use of ultrasound in orthopaedics: a bibliometric analysis of global research. Am J Transl Res. 2021;13:9892–9911. [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou Q, Wu F, Zhao M, Yang M. Bibliometric evaluation of 2012-2020 publications on ferroptosis in cancer treatment. Front Cell Dev Biol. 2021;9:793347. doi: 10.3389/fcell.2021.793347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu L, Tang F, Wang Y, Cai Q, Tang S, Xia D, Xu X, Lu X. Research progress of pre-hospital emergency during 2000-2020: a bibliometric analysis. Am J Transl Res. 2021;13:1109–1124. [PMC free article] [PubMed] [Google Scholar]
- 18.Synnestvedt MB, Chen C, Holmes JH. CiteSpace II: visualization and knowledge discovery in bibliographic databases. AMIA Annu Symp Proc. 2005;2005:724–728. [PMC free article] [PubMed] [Google Scholar]
- 19.van Eck NJ, Waltman L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics. 2010;84:523–538. doi: 10.1007/s11192-009-0146-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou F, Zhang T, Jin Y, Ma Y, Xian Z, Zeng M, Yu G. Emerging trends and research foci in allergic rhinitis immunotherapy from 2002 to 2021: a bibliometric and visualized study. Am J Transl Res. 2022;14:4457–4476. [PMC free article] [PubMed] [Google Scholar]
- 21.Zhong D, Li Y, Huang Y, Hong X, Li J, Jin R. Molecular mechanisms of exercise on cancer: a bibliometrics study and visualization analysis via CiteSpace. Front Mol Biosci. 2021;8:797902. doi: 10.3389/fmolb.2021.797902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Epelman S, Lavine KJ, Beaudin AE, Sojka DK, Carrero JA, Calderon B, Brija T, Gautier EL, Ivanov S, Satpathy AT, Schilling JD, Schwendener R, Sergin I, Razani B, Forsberg EC, Yokoyama WM, Unanue ER, Colonna M, Randolph GJ, Mann DL. Embryonic and adult-derived resident cardiac macrophages are maintained through distinct mechanisms at steady state and during inflammation. Immunity. 2014;40:91–104. doi: 10.1016/j.immuni.2013.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ortega-Gómez A, Perretti M, Soehnlein O. Resolution of inflammation: an integrated view. EMBO Mol Med. 2013;5:661–674. doi: 10.1002/emmm.201202382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liao X, Sluimer JC, Wang Y, Subramanian M, Brown K, Pattison JS, Robbins J, Martinez J, Tabas I. Macrophage autophagy plays a protective role in advanced atherosclerosis. Cell Metab. 2012;15:545–553. doi: 10.1016/j.cmet.2012.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Poon IK, Lucas CD, Rossi AG, Ravichandran KS. Apoptotic cell clearance: basic biology and therapeutic potential. Nat Rev Immunol. 2014;14:166–180. doi: 10.1038/nri3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arandjelovic S, Ravichandran KS. Phagocytosis of apoptotic cells in homeostasis. Nat Immunol. 2015;16:907–917. doi: 10.1038/ni.3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gheibi Hayat SM, Bianconi V, Pirro M, Sahebkar A. Efferocytosis: molecular mechanisms and pathophysiological perspectives. Immunol Cell Biol. 2019;97:124–133. doi: 10.1111/imcb.12206. [DOI] [PubMed] [Google Scholar]
- 28.Tajbakhsh A, Rezaee M, Kovanen PT, Sahebkar A. Efferocytosis in atherosclerotic lesions: malfunctioning regulatory pathways and control mechanisms. Pharmacol Ther. 2018;188:12–25. doi: 10.1016/j.pharmthera.2018.02.003. [DOI] [PubMed] [Google Scholar]
- 29.Morioka S, Maueröder C, Ravichandran KS. Living on the edge: efferocytosis at the interface of homeostasis and pathology. Immunity. 2019;50:1149–1162. doi: 10.1016/j.immuni.2019.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vago JP, Amaral FA, van de Loo FAJ. Resolving inflammation by TAM receptor activation. Pharmacol Ther. 2021;227:107893. doi: 10.1016/j.pharmthera.2021.107893. [DOI] [PubMed] [Google Scholar]
- 31.Segawa K, Nagata S. An apoptotic ‘eat me’ signal: phosphatidylserine exposure. Trends Cell Biol. 2015;25:639–650. doi: 10.1016/j.tcb.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 32.Kourtzelis I, Hajishengallis G, Chavakis T. Phagocytosis of apoptotic cells in resolution of inflammation. Front Immunol. 2020;11:553. doi: 10.3389/fimmu.2020.00553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dalli J, Serhan CN. Specific lipid mediator signatures of human phagocytes: microparticles stimulate macrophage efferocytosis and pro-resolving mediators. Blood. 2012;120:e60–72. doi: 10.1182/blood-2012-04-423525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dalli J, Serhan CN. Pro-resolving mediators in regulating and conferring macrophage function. Front Immunol. 2017;8:1400. doi: 10.3389/fimmu.2017.01400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao J, Zhang W, Wu T, Wang H, Mao J, Liu J, Zhou Z, Lin X, Yan H, Wang Q. Efferocytosis in the central nervous system. Front Cell Dev Biol. 2021;9:773344. doi: 10.3389/fcell.2021.773344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Trzeciak A, Wang YT, Perry JSA. First we eat, then we do everything else: the dynamic metabolic regulation of efferocytosis. Cell Metab. 2021;33:2126–2141. doi: 10.1016/j.cmet.2021.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.A-Gonzalez N, Hidalgo A. Nuclear receptors and clearance of apoptotic cells: stimulating the macrophage’s appetite. Front Immunol. 2014;5:211. doi: 10.3389/fimmu.2014.00211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Viaud M, Ivanov S, Vujic N, Duta-Mare M, Aira LE, Barouillet T, Garcia E, Orange F, Dugail I, Hainault I, Stehlik C, Marchetti S, Boyer L, Guinamard R, Foufelle F, Bochem A, Hovingh KG, Thorp EB, Gautier EL, Kratky D, Dasilva-Jardine P, Yvan-Charvet L. Lysosomal cholesterol hydrolysis couples efferocytosis to anti-inflammatory oxysterol production. Circ Res. 2018;122:1369–1384. doi: 10.1161/CIRCRESAHA.117.312333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang S, Weinberg S, DeBerge M, Gainullina A, Schipma M, Kinchen JM, Ben-Sahra I, Gius DR, Yvan-Charvet L, Chandel NS, Schumacker PT, Thorp EB. Efferocytosis fuels requirements of fatty acid oxidation and the electron transport chain to polarize macrophages for tissue repair. Cell Metab. 2019;29:443–456. e445. doi: 10.1016/j.cmet.2018.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Proto JD, Doran AC, Gusarova G, Yurdagul A Jr, Sozen E, Subramanian M, Islam MN, Rymond CC, Du J, Hook J, Kuriakose G, Bhattacharya J, Tabas I. Regulatory T cells promote macrophage efferocytosis during inflammation resolution. Immunity. 2018;49:666–677. doi: 10.1016/j.immuni.2018.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Greenlee-Wacker MC. Clearance of apoptotic neutrophils and resolution of inflammation. Immunol Rev. 2016;273:357–370. doi: 10.1111/imr.12453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Singhal A, Kumar S. Neutrophil and remnant clearance in immunity and inflammation. Immunology. 2022;165:22–43. doi: 10.1111/imm.13423. [DOI] [PubMed] [Google Scholar]
- 43.Sexton DW, Blaylock MG, Walsh GM. Human alveolar epithelial cells engulf apoptotic eosinophils by means of integrin- and phosphatidylserine receptor-dependent mechanisms: a process upregulated by dexamethasone. J Allergy Clin Immunol. 2001;108:962–969. doi: 10.1067/mai.2001.119414. [DOI] [PubMed] [Google Scholar]
- 44.Rottner M, Freyssinet JM, Martínez MC. Mechanisms of the noxious inflammatory cycle in cystic fibrosis. Respir Res. 2009;10:23. doi: 10.1186/1465-9921-10-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eltboli O, Bafadhel M, Hollins F, Wright A, Hargadon B, Kulkarni N, Brightling C. COPD exacerbation severity and frequency is associated with impaired macrophage efferocytosis of eosinophils. BMC Pulm Med. 2014;14:112. doi: 10.1186/1471-2466-14-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Simpson JL, Gibson PG, Yang IA, Upham J, James A, Reynolds PN, Hodge S. Impaired macrophage phagocytosis in non-eosinophilic asthma. Clin Exp Allergy. 2013;43:29–35. doi: 10.1111/j.1365-2222.2012.04075.x. [DOI] [PubMed] [Google Scholar]
- 47.Kojima Y, Weissman IL, Leeper NJ. The role of efferocytosis in atherosclerosis. Circulation. 2017;135:476–489. doi: 10.1161/CIRCULATIONAHA.116.025684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moore KJ, Sheedy FJ, Fisher EA. Macrophages in atherosclerosis: a dynamic balance. Nat Rev Immunol. 2013;13:709–721. doi: 10.1038/nri3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moore KJ, Tabas I. Macrophages in the pathogenesis of atherosclerosis. Cell. 2011;145:341–355. doi: 10.1016/j.cell.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jarr KU, Kojima Y, Weissman IL, Leeper NJ. 2021 Jeffrey M. Hoeg award lecture: defining the role of efferocytosis in cardiovascular disease: a focus on the cd47 (cluster of differentiation 47) Axis. Arterioscler Thromb Vasc Biol. 2022;42:e145–e154. doi: 10.1161/ATVBAHA.122.317049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Abdolmaleki F, Farahani N, Gheibi Hayat SM, Pirro M, Bianconi V, Barreto GE, Sahebkar A. The role of efferocytosis in autoimmune diseases. Front Immunol. 2018;9:1645. doi: 10.3389/fimmu.2018.01645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bukong TN, Cho Y, Iracheta-Vellve A, Saha B, Lowe P, Adejumo A, Furi I, Ambade A, Gyongyosi B, Catalano D, Kodys K, Szabo G. Abnormal neutrophil traps and impaired efferocytosis contribute to liver injury and sepsis severity after binge alcohol use. J Hepatol. 2018;69:1145–1154. doi: 10.1016/j.jhep.2018.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zheng C, Sui B, Zhang X, Hu J, Chen J, Liu J, Wu D, Ye Q, Xiang L, Qiu X, Liu S, Deng Z, Zhou J, Liu S, Shi S, Jin Y. Apoptotic vesicles restore liver macrophage homeostasis to counteract type 2 diabetes. J Extracell Vesicles. 2021;10:e12109. doi: 10.1002/jev2.12109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhou Y, Yao Y, Deng Y, Shao A. Regulation of efferocytosis as a novel cancer therapy. Cell Commun Signal. 2020;18:71. doi: 10.1186/s12964-020-00542-9. [DOI] [PMC free article] [PubMed] [Google Scholar]