Figure 6.
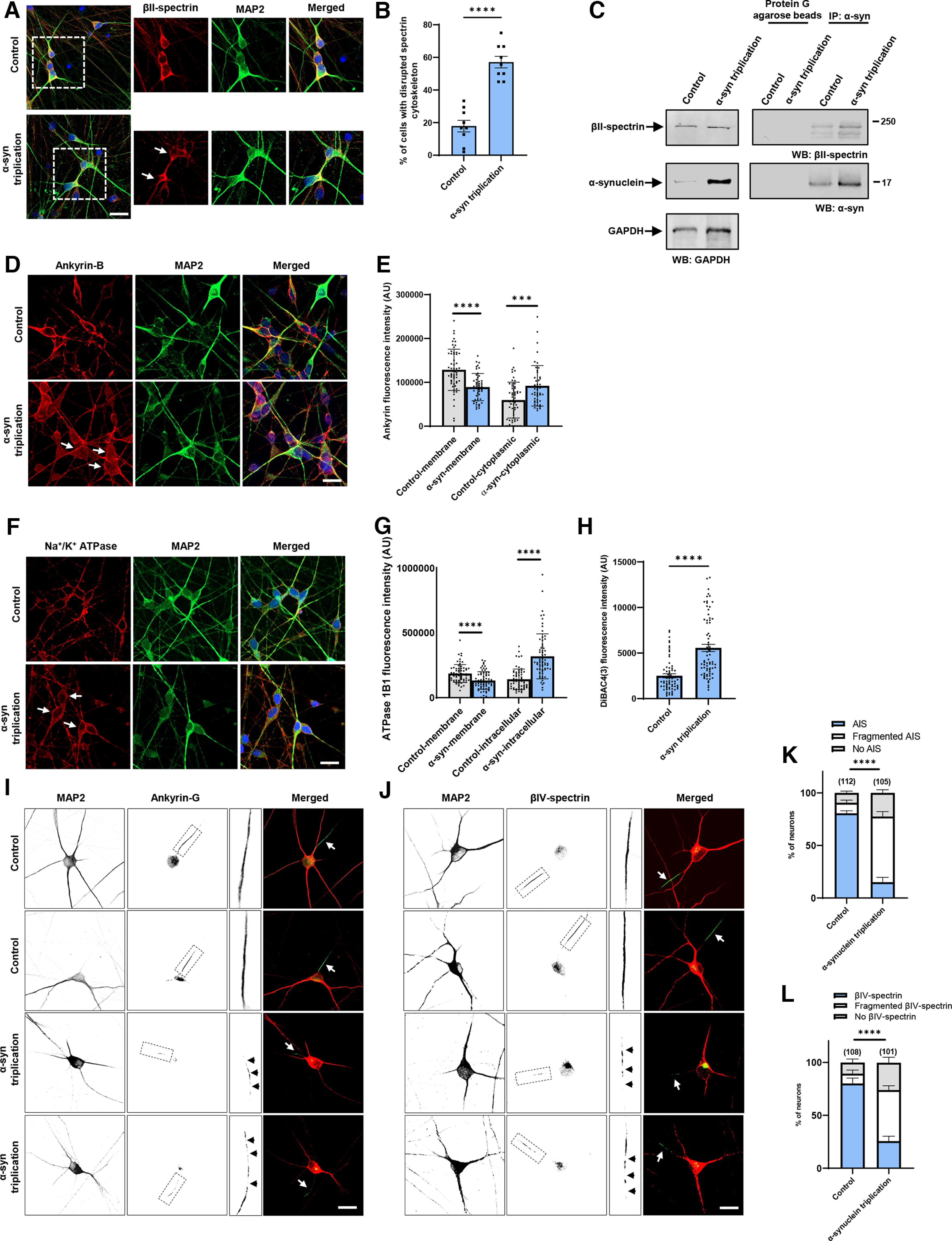
Increased expression of α-synuclein leads to disruption of the spectrin cytoskeleton and mislocalization of ankyrin and Na+/K+ ATPase in human neurons. A, B, The normal βII-spectrin immunoreactive subplasmalemmal network (red) is disrupted by increased expression of human α-synuclein in MAP2-positive (green) α-synuclein triplication patient neurons compared with isogenic control neurons. Arrows indicate cells with reduced βII-spectrin staining. n = 9. C, Immunoprecipitation of α-synuclein in control and α-synuclein triplication patient-derived neurons shows an association between α-synuclein and βII-spectrin. The blot is reprobed with an antibody to GAPDH to illustrate equivalent protein levels. D, E, Immunofluorescence microscopy reveals subplasmalemmal staining pattern for ankyrin-B (red) in MAP2-positive (green) control neurons and increased cytoplasmic staining in patient neurons (arrows). F, G, Immunostaining for Na+/K+ ATPase (red) shows a plasma membrane staining pattern in MAP2-positive (green) control neurons increased cytoplasmic staining in α-synuclein triplication patient neurons (arrows). H, Loss of plasma membrane polarization as monitored by DiBAC4(3) in α-synuclein triplication patient neurons compared with controls. B, E, G, H, Data are mean ± SEM. p values determined with two-tailed t test. I–L, Immunostaining for ankyrin-G (I) or βIV-spectrin (J) identifies intact axon initial segments in most MAP2-positive control neurons (arrows) and increased numbers of neurons with loss or fragmentation of the axon initial segment (arrowheads) in α-synuclein triplication patient neurons, as quantified in K, L. Assays were performed at 21 DIV. Scale bars, 25 µm. Data are mean ± SEM. p values determined with two-way ANOVA with Bonferroni post hoc test.
