Abstract
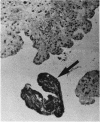
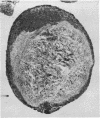
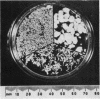
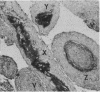
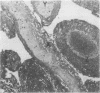
The incidence of rice bodies (RB) in synovial effusions has been studied in 36 patients with rheumatoid arthritis (RA) and in 12 patients with seronegative inflammatory arthritis (7 cases of Still's disease, 3 of psoriatic arthritis, and 2 of ankylosing spondylitis). In the RA group 50 joints were aspirated before and after saline lavage with a specially designed wide-bore needle. RB were found in 72% overall of the joints studied in this group, 14% on initial simple aspiration and an additional 58% after lavage. In contrast no rice bodies were found in 31 aspirations with lavage by an identical technique in the 12 patients with seronegative synovitis. The RB in RA synovitis occurred both early and late in the course of the disease and were not related to the severity of clinical or radiological changes. However, removal of rice bodies was accompanied by clinical improvement and reduction of synovitis. Macroscopically RB varied in shape and size, some being so large as to preclude effective removal by needles of the gauge customarily employed for joint aspirations. Microscopically the majority of RB were composed of coarsely reticular material reacting immunologically with antifibrinogen and antifibronectin and containing mononuclear cells. Some showed vacuolation suggestive of fibrinolysis, but many showed organisation like that seen in established connective tissues, with the formation of mature collagen, reticulin, and elastin. These findings are discussed in relation to the origin, development, and significance of rice bodies in rheumatoid synovitis.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASTRUP T., SJOLIN K. E. Thromboplastic and fibrinolytic activity of human synovial membrane and fibrous capsular tissue. Proc Soc Exp Biol Med. 1958 Apr;97(4):852–853. doi: 10.3181/00379727-97-23898. [DOI] [PubMed] [Google Scholar]
- Albrecht M., Marinetti G. V., Jacox R. F., Vaughan J. H. A biochemical and electron microscopy study of rice bodies from rheumatoid patients. Arthritis Rheum. 1965 Dec;8(6):1053–1063. doi: 10.1002/art.1780080605. [DOI] [PubMed] [Google Scholar]
- Andersen R. B., Gormsen J. Fibrinolytic and fibrin stabilizing activity of synovial membranes. Ann Rheum Dis. 1970 May;29(3):287–293. doi: 10.1136/ard.29.3.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnhart M. I., Riddle J. M., Bluhm G. B., Quintana C. Fibrin promotion and lysis in arthritic joints. Ann Rheum Dis. 1967 May;26(3):206–218. doi: 10.1136/ard.26.3.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg E., Wainwright R., Barton B., Puchtler H., McDonald T. On the nature of rheumatoid rice bodies: an immunologic, histochemical, and electron microscope study. Arthritis Rheum. 1977 Sep-Oct;20(7):1343–1349. doi: 10.1002/art.1780200707. [DOI] [PubMed] [Google Scholar]
- Berger H., Jr Secretion of plasminogen activator by rheumatoid and nonrheumatoid synovial cells in culture. Arthritis Rheum. 1977 Jul-Aug;20(6):1198–1205. doi: 10.1002/art.1780200607. [DOI] [PubMed] [Google Scholar]
- Blumberg P. M., Robbins P. W. Effect of proteases on activation of resting chick embryo fibroblasts and on cell surface proteins. Cell. 1975 Oct;6(2):137–147. doi: 10.1016/0092-8674(75)90004-5. [DOI] [PubMed] [Google Scholar]
- Caughey D. E., Highton T. C. Components of the fibrinolytic system in synovial joints. Normal bovine compared with normal and abnormal human synovial joints. Ann Rheum Dis. 1967 Jul;26(4):297–305. doi: 10.1136/ard.26.4.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemmensen I., Donde R., Andersen R. B. The primary plasmin inhibitor in rheumatoid synovial fluid. Arthritis Rheum. 1977 Sep-Oct;20(7):1354–1358. doi: 10.1002/art.1780200709. [DOI] [PubMed] [Google Scholar]
- Glynn L. E. The chronicity of inflammation and its significance in rheumatoid arthritis. Ann Rheum Dis. 1968 Mar;27(2):105–121. doi: 10.1136/ard.27.2.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon H., Sweets H. H. A Simple Method for the Silver Impregnation of Reticulum. Am J Pathol. 1936 Jul;12(4):545–552.1. [PMC free article] [PubMed] [Google Scholar]
- HEROVICI C. [Picropolychrome: histological staining technic intended for the study of normal and pathological connective tissue]. Rev Fr Etud Clin Biol. 1963 Jan;8:88–89. [PubMed] [Google Scholar]
- LENDRUM A. C., FRASER D. S., SLIDDERS W., HENDERSON R. Studies on the character and staining of fibrin. J Clin Pathol. 1962 Sep;15:401–413. doi: 10.1136/jcp.15.5.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linder E., Stenman S., Lehto V. P., Vaheri A. Distribution of fibronectin in human tissues and relationship to other connective tissue components. Ann N Y Acad Sci. 1978 Jun 20;312:151–159. doi: 10.1111/j.1749-6632.1978.tb16800.x. [DOI] [PubMed] [Google Scholar]
- McDonald J. A., Baum B. J., Rosenberg D. M., Kelman J. A., Brin S. C., Crystal R. G. Destruction of a major extracellular adhesive glycoprotein (fibronectin) of human fibroblasts by neutral proteases from polymorphonuclear leukocyte granules. Lab Invest. 1979 Mar;40(3):350–357. [PubMed] [Google Scholar]
- Miller P. J. An elastin stain. Med Lab Technol. 1971 Apr;28(2):148–149. [PubMed] [Google Scholar]
- Ruoslahti E., Vaheri A. Interaction of soluble fibroblast surface antigen with fribrinogen and fibrin. J Exp Med. 1975 Feb 1;141(2):497–501. doi: 10.1084/jem.141.2.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saba T. M., Blumenstock F. A., Weber P., Kaplan J. E. Physiologic role of cold-insoluble globulin in systemic host defense: implications of its characterization as the opsonic alpha 2-surface-binding glycoprotein. Ann N Y Acad Sci. 1978 Jun 20;312:43–55. doi: 10.1111/j.1749-6632.1978.tb16792.x. [DOI] [PubMed] [Google Scholar]
- Scott D. L., Wainwright A. C., Walton K. W., Williamson N. Significance of fibronectin in rheumatoid arthritis and osteoarthrosis. Ann Rheum Dis. 1981 Apr;40(2):142–153. doi: 10.1136/ard.40.2.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van De Putte L. B., Hegt V. N., Overbeek T. E. Activators and inhibitors of fibrinolysis in rheumatoid and nonrheumatoid synovial membranes. A histochemical study. Arthritis Rheum. 1977 Mar;20(2):671–678. doi: 10.1002/art.1780200206. [DOI] [PubMed] [Google Scholar]