Abstract
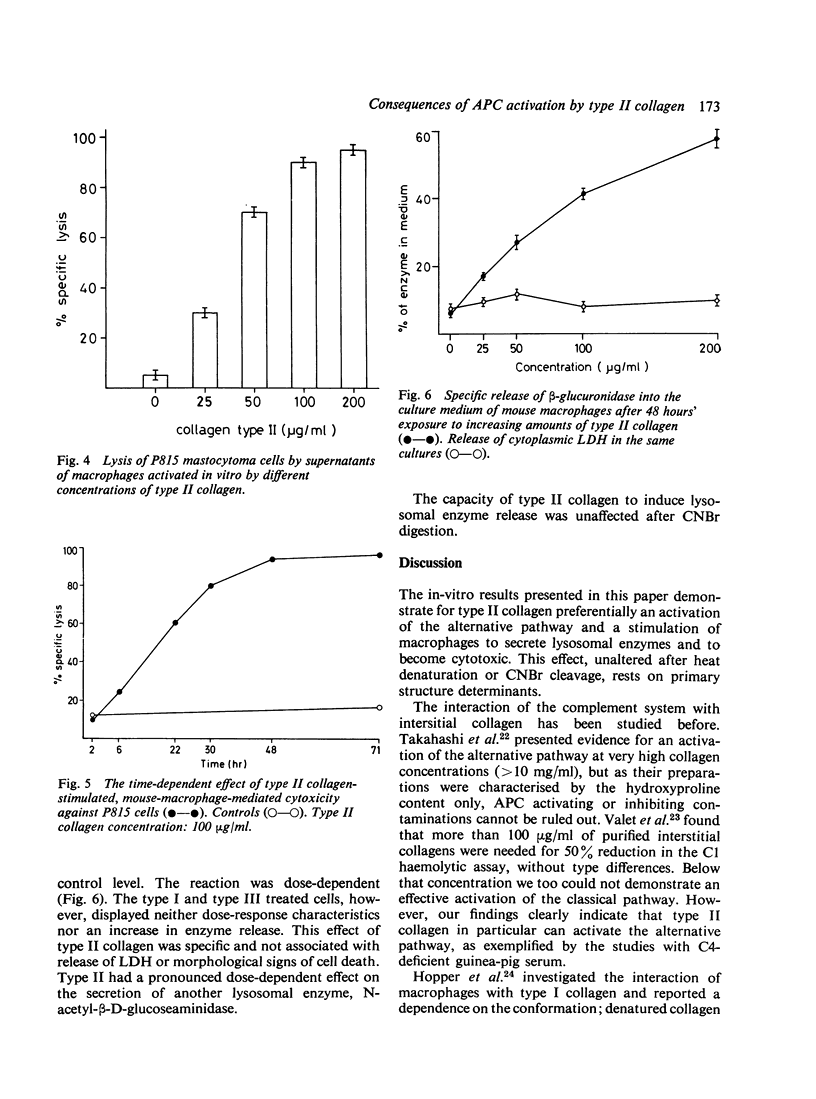
We studied the effect of human interstitial collagen types I, II, and III on serum-free cultured mouse macrophages and on the complement classical and alternative pathways in human and guinea-pig serum. Type II collagen produced a dose-dependent consumption and conversion of C3 and factor B both in the homologous and in the heterologous system. This effect on the alternative pathway was reproduced in genetically C4-deficient guinea-pig serum and could be triggered by native, triple helical type II molecules, by their component alpha chains, and the CNBr peptide mixture. Addition of type II collagen to the mouse macrophage cultures induced not only a dose- and time-dependent secretion of lysosomal enzymes, but also the generation of a supernatant factor cytotoxic for mouse mastocytoma P 815 cells. Collagen of types I and III were conspicuously less active or inactive in all assays. The studies demonstrate properties of the collagen specific for cartilage which, on a molecular level, suggest its direct, local participation in the production and perpetuation of rheumatoid arthritis.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BITTER T., MUIR H. M. A modified uronic acid carbazole reaction. Anal Biochem. 1962 Oct;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- Bitter-Suermann D., Becker S., Meuer S., Schorlemmer H. U., Hadding U., Andreatta R. Comparative study on biological effects of the guinea pig complement-peptide C3a and C3a-related synthetic oligopeptides. Mol Immunol. 1980 Oct;17(10):1257–1261. doi: 10.1016/0161-5890(80)90022-x. [DOI] [PubMed] [Google Scholar]
- Bitter-Suermann D., Hadding U., Melchert F., Wellensiek H. J. Independent and consecutive action of the complement components C5, C6 and C7 in immune hemolysis. I. Preparation of EAC1-5 with purified guinea pig C3 and C5. Immunochemistry. 1970 Dec;7(12):955–965. doi: 10.1016/0019-2791(70)90002-9. [DOI] [PubMed] [Google Scholar]
- Carmichael D. J., Gillard G. C., Lowther D. A., Handley C. J., Santer V. B. Carrageenin-induced arthritis. IV. Rate changes in cartilage matrix proteoglycan synthesis. Arthritis Rheum. 1977 Apr;20(3):834–840. doi: 10.1002/art.1780200313. [DOI] [PubMed] [Google Scholar]
- Courtenay J. S., Dallman M. J., Dayan A. D., Martin A., Mosedale B. Immunisation against heterologous type II collagen induces arthritis in mice. Nature. 1980 Feb 14;283(5748):666–668. doi: 10.1038/283666a0. [DOI] [PubMed] [Google Scholar]
- Epstein E. H., Jr (Alpha1(3))3 human skin collagen. Release by pepsin digestion and preponderance in fetal life. J Biol Chem. 1974 May 25;249(10):3225–3231. [PubMed] [Google Scholar]
- Furthmayr H., Timpl R. Characterization of collagen peptides by sodium dodecylsulfate-polyacrylamide electrophoresis. Anal Biochem. 1971 Jun;41(2):510–516. doi: 10.1016/0003-2697(71)90173-4. [DOI] [PubMed] [Google Scholar]
- Glynn L. E. Experimental models and etiology of inflammatory rheumatic diseases. Scand J Rheumatol Suppl. 1975;12:55–62. [PubMed] [Google Scholar]
- Harris E. D., Jr, Glauert A. M., Murley A. H. Intracellular collagen fibers at the pannus-cartilage junction in rheumatoid arthritis. Arthritis Rheum. 1977 Mar;20(2):657–665. doi: 10.1002/art.1780200204. [DOI] [PubMed] [Google Scholar]
- Hopper K. E., Adelmann B. C., Gentner G., Gay S. Recongnition by guinea-pig peritoneal exudate cells of conformationally different states of the collagen molecule. Immunology. 1976 Feb;30(2):249–259. [PMC free article] [PubMed] [Google Scholar]
- Ishikawa H., Ziff M. Electron microscopic observations of immunoreactive cells in the rheumatoid synovial membrane. Arthritis Rheum. 1976 Jan-Feb;19(1):1–14. doi: 10.1002/art.1780190101. [DOI] [PubMed] [Google Scholar]
- Keystone E. C., Schorlemmer H. U., Pope C., Allison A. C. Zymosan-induced arthritis: a model of chronic proliferative arthritis following activation of the alternative pathway of complement. Arthritis Rheum. 1977 Sep-Oct;20(7):1396–1401. doi: 10.1002/art.1780200714. [DOI] [PubMed] [Google Scholar]
- Klaus G. G., Humphrey J. H. The generation of memory cells. I. The role of C3 in the generation of B memory cells. Immunology. 1977 Jul;33(1):31–40. [PMC free article] [PubMed] [Google Scholar]
- Lohmander S. Proteoglycans of guinea-pig coastal cartilage. Fractionation and characterization. Eur J Biochem. 1975 Sep 15;57(2):549–559. doi: 10.1111/j.1432-1033.1975.tb02330.x. [DOI] [PubMed] [Google Scholar]
- Miller E. J. Isolation and characterization of a collagen from chick cartilage containing three identical alpha chains. Biochemistry. 1971 Apr 27;10(9):1652–1659. doi: 10.1021/bi00785a024. [DOI] [PubMed] [Google Scholar]
- Miller E. J. Structural studies on cartilage collagen employing limited cleavage and solubilization with pepsin. Biochemistry. 1972 Dec 19;11(26):4903–4909. doi: 10.1021/bi00776a005. [DOI] [PubMed] [Google Scholar]
- Munthe E., Hoyeraal H. M., Froland S. S., Mellbye O. J., Kåss E., Natvig J. B. Evidence for complement activation by the alternate pathway in the arthritis of hypogammaglobulinemic patients. Rheumatology. 1975;6:43–51. [PubMed] [Google Scholar]
- Müller W., Hanauske-Abel H., Loos M. Biosynthesis of the first component of complement by human and guinea pig peritoneal macrophages: evidence for an independent production of the C1 subunits. J Immunol. 1978 Oct;121(4):1578–1584. [PubMed] [Google Scholar]
- Postlethwaite A. E., Kang A. H. Collagen-and collagen peptide-induced chemotaxis of human blood monocytes. J Exp Med. 1976 Jun 1;143(6):1299–1307. doi: 10.1084/jem.143.6.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossen R. D., Brewer E. J., Sharp R. M., Ott J., Templeton J. W. Familial rheumatoid arthritis: linkage of HLA to disease susceptibility locus in four families where proband presented with juvenile rheumatoid arthritis. J Clin Invest. 1980 Mar;65(3):629–642. doi: 10.1172/JCI109708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruddy S., Fearon D. T., Austen K. F. Depressed synovial fluid levels of properdin and properdin factor B in patients with rheumatoid arthritis. Arthritis Rheum. 1975 Jul-Aug;18(4):289–295. doi: 10.1002/art.1780180401. [DOI] [PubMed] [Google Scholar]
- Schorlemmer H. U., Bitter-Suermann D., Allison A. C. Complement activation by the alternative pathway and macrophage enzyme secretion in the pathogenesis of chronic inflammation. Immunology. 1977 Jun;32(6):929–940. [PMC free article] [PubMed] [Google Scholar]
- Schwab J. H., Cromartie W. J., Ohanian S. H., Craddock J. G. Association of experimental chronic arthritis with the persistence of group A streptococcal cell walls in the articular tissue. J Bacteriol. 1967 Nov;94(5):1728–1735. doi: 10.1128/jb.94.5.1728-1735.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shtacher G., Maayan R., Feinstein G. Proteinase inhibitors in human synovial fluid. Biochim Biophys Acta. 1973 Mar 23;303(1):138–147. doi: 10.1016/0005-2795(73)90155-4. [DOI] [PubMed] [Google Scholar]
- Stephens R. W., Ghosh P., Taylor T. K., Gale C. A., Swann J. C., Robinson R. G., Webb J. The origins and relative distribution of polysaccharidases in rheumatoid and osteoarthritic fluids. J Rheumatol. 1975 Dec;2(4):393–400. [PubMed] [Google Scholar]
- Stuart J. M., Cremer M. A., Dixit S. N., Kang A. H., Townes A. S. Collagen-induced arthritis in rats. Comparison of vitreous and cartilage-derived collagens. Arthritis Rheum. 1979 Apr;22(4):347–352. doi: 10.1002/art.1780220406. [DOI] [PubMed] [Google Scholar]
- Takahashi M., KawachiTakahashi S., Matsuura M. Interaction of collagen with serum complement: inhibition of complement-mediated hemolysis. Int Arch Allergy Appl Immunol. 1975;48(5):642–652. doi: 10.1159/000231352. [DOI] [PubMed] [Google Scholar]
- Trentham D. E., Dynesius R. A., David J. R. Passive transfer by cells of type II collagen-induced arthritis in rats. J Clin Invest. 1978 Aug;62(2):359–366. doi: 10.1172/JCI109136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trentham D. E., Townes A. S., Kang A. H. Autoimmunity to type II collagen an experimental model of arthritis. J Exp Med. 1977 Sep 1;146(3):857–868. doi: 10.1084/jem.146.3.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trentham D. E., Townes A. S., Kang A. H., David J. R. Humoral and cellular sensitivity to collagen in type II collagen-induced arthritis in rats. J Clin Invest. 1978 Jan;61(1):89–96. doi: 10.1172/JCI108929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zvaifler N. J. Rheumatoid synovitis. An extravascular immune complex disease. Arthritis Rheum. 1974 May-Jun;17(3):297–305. doi: 10.1002/art.1780170315. [DOI] [PubMed] [Google Scholar]