Figure 3. SAYSD1 preferentially engages translocation-stalled nascent chain-ribosome complexes in a UFMylation dependent manner.
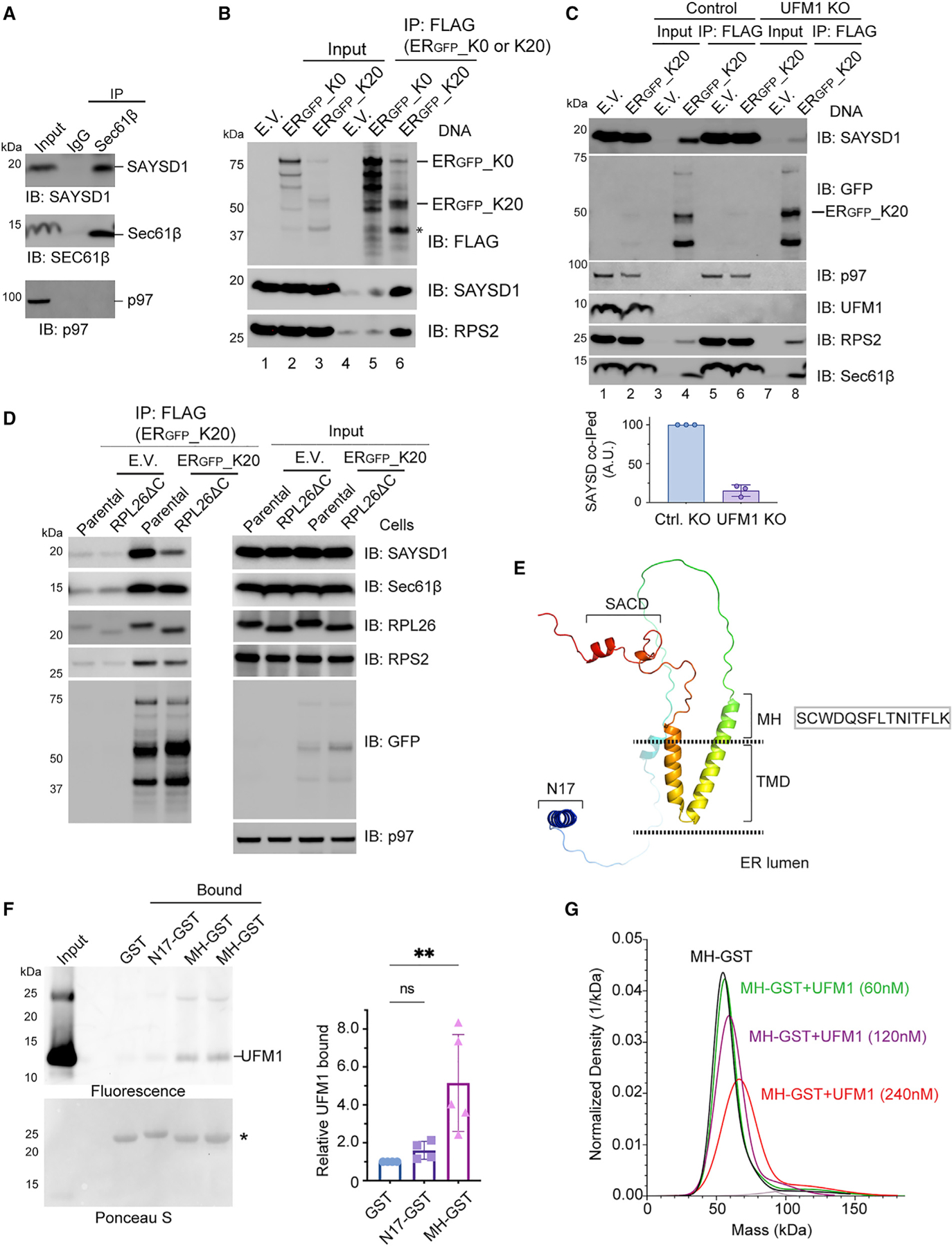
(A) Co-immunoprecipitation of endogenous SAYSD1 with the Sec61 translocon component Sec61β from 293T cells.
(B) SAYSD1 associates with ERGFP_K20 in a translation-stalling-dependent manner. 293T cells transfected with an empty vector (EV), ERGFP_K20, or ERGFP_K0 were subject to immunoprecipitation by FLAG beads. The asterisk indicates the stalled ERGFP_K20 species.
(C) The association of SAYSD1 with ERGFP_K20 is dependent on UFM1. Control or UFM1 CRISPR KO cells transfected as indicated were subject to FLAG pull-down and immunoblotting. The graph shows the quantification of three independent experiments.
(D) The association of SAYSD1 with ERGFP_K20 requires the RPL26 C tail. As in (C) except that cells that have the endogenous RPL26 C-terminal tail deleted by CRISPR-mediated homologous recombination were used.
(E) The predicted SAYSD1 structure by AlphaFold. SACD, SAYSvFN-containing domain; TMD, transmembrane domain.
(F) SAYSD1 binds the MH domain of UFM1 directly. The indicated that GST-tagged protein or GST immobilized on glutathione beads were incubated with Atto565-labeled UFM1. The precipitated proteins were SDS-PAGE fractionated and detected by a fluorescence scanner (top) or Ponceau S staining (bottom). The graph shows the quantification of 4 independent experiments. Error bars, means ± SD; **p < 0.01 by unpaired Student’s t test. n = 4.
(G) Mass photometry shows a direction interaction of MH-GST with UFM1.
