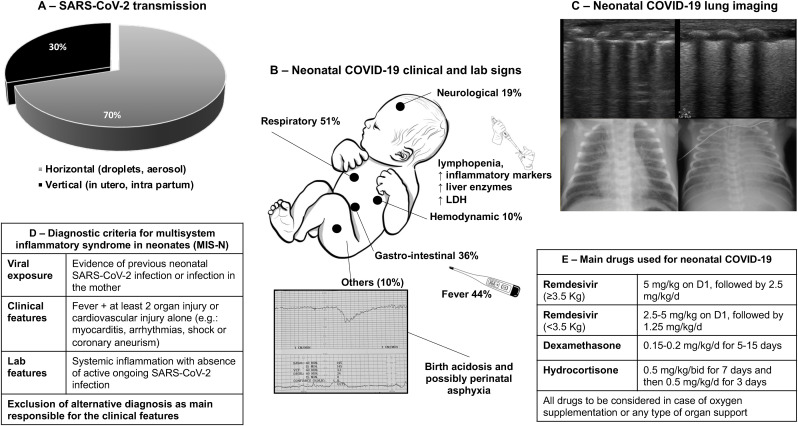
Fig. 5.
Main clinical features, laboratory and imaging abnormalities of neonatal COVID-19, as well as diagnostic criteria for MIS-N (adapted from Ref. [96]). Panel A shows the distribution of SARS-CoV-2 transmission routes: black and grey areas indicate vertical and horizontal transmission, respectively. Panel B reports the clinical and laboratory signs observed in neonates with clinically evident SARS-CoV-2 infection (at least one clinical sign develops in approximately 50% of neonatal infections; multiple signs are possible in a patient), as reported by Ref. [18]. Panel C illustrates lung ultrasound and conventional radiology findings in neonates with respiratory involvement: images on the left- and on the right-side show a typical interstitial and alveolar pattern, respectively: the interstitial is more common, but it may evolve in alveolar pattern; neonatal ARDS [106] has been diagnosed in the rare severest cases. Panel D reports the diagnostic criteria for multisystem inflammatory syndrome in neonates (MIS-N), as proposed by Ref. [94]. Panel E summarizes drug doses for severe/critical neonatal COVID-19 and MIS-N treatment. Remdesivir (VEKLURY®, Gilead, Foster City-CA, USA) has been approved by FDA and EMA for the treatment of neonates weighting at least 3 Kg; same case reports have described its use, at lower doses, also for smaller neonates [96]. Dexamethasone and hydrocortisone are the two steroids used in case series of neonatal COVID-19 and MIS-N have also been used together with intravenous immunoglobulins-G with classical dosing.