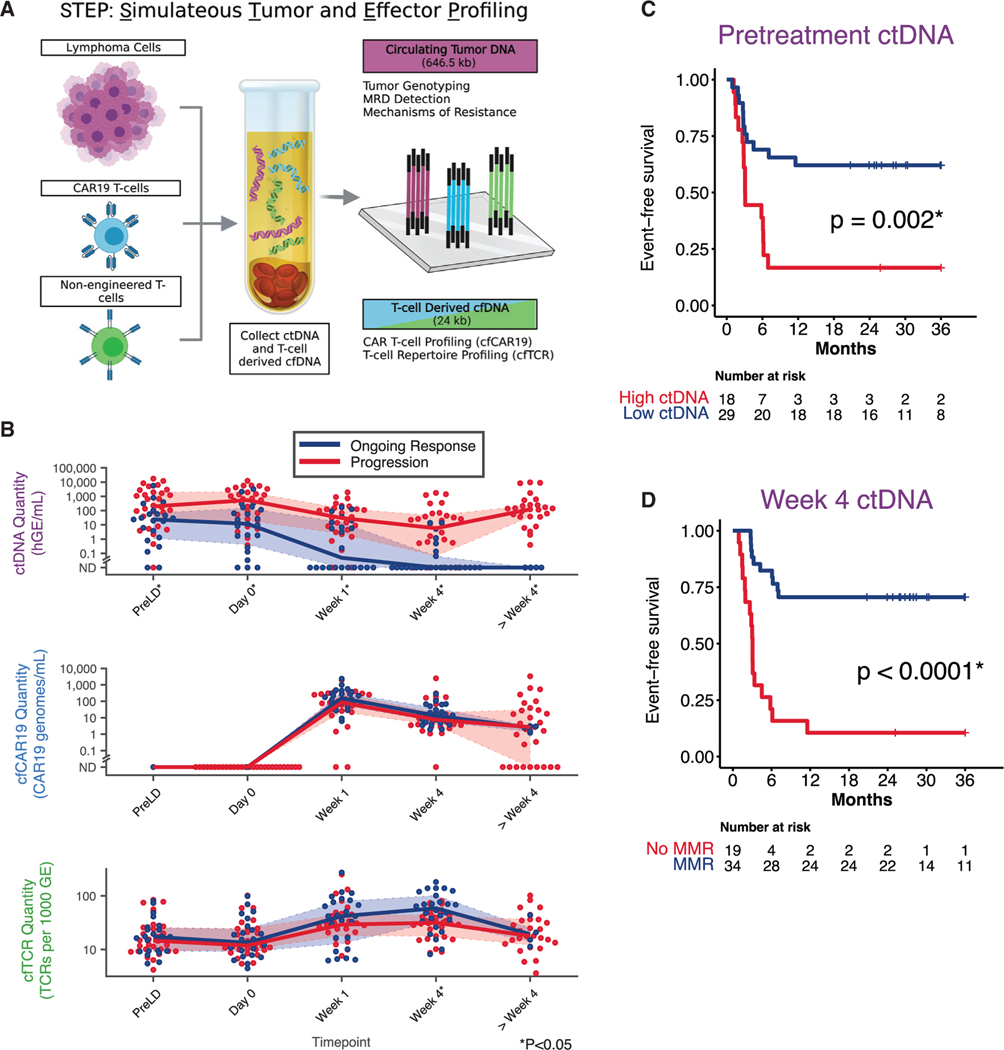
Figure 2. STEP platform and impact of ctDNA molecular thresholds on outcome.
(A) Illustration summarizing the strategy through which ctDNA, cfCAR19, and cfTCR are simultaneously profiled from a plasma sample using the simultaneous tumor and effector profiling (STEP) platform.
(B) Dynamic changes in ctDNA (top), cfCAR19 (middle), and cfTCR levels (bottom) following CAR19 infusion in patients who progress (red), and those who achieve an ongoing response (blue). Wilcoxon rank-sum test used to compare variables at each noted time point.
(C and D) Kaplan-Meier estimates show EFS for patients stratified by pretreatment (day 0; C) or dynamic (week 4; D) ctDNA levels using optimized molecular thresholds in the discovery cohort. High ctDNA defined as ≥2.5 log10hGE/mL. MMR defined as ≥2.5 log decrease in ctDNA level relative to day 0. hGE, haploid genome equivalent; SABER, sequence affinity capture and analysis by enumeration of cell-free receptors; MMR, major molecular response, GE, genome equivalent. *p < 0.05. See also Figure S1.