Summary
Background
The recent Sudan virus (SUDV) outbreak in Uganda highlights the need for rapid response capabilities, including development of vaccines against emerging viruses with high public health impact. We aimed to develop a Sudan virus-specific vaccine suitable for emergency use during outbreaks.
Methods
We generated and characterised a vesicular stomatitis virus (VSV)-based vaccine, VSV- SUDV, and evaluated the protective efficacy following a single-dose vaccination against lethal SUDV infection in non-human primates (NHPs). We used male and female cynomolgus macaques (n=11) aged 6–11 years and weighing 3·8–9·0 kg. Animals received a 1 mL intramuscular injection for vaccination containing either 1 × 107 plaque forming units (PFU) VSV-SUDV or 1 × 107 PFU of a VSV-based vaccine against Marburg virus (control; five NHPs). NHPs were challenged intramuscularly 28 days after vaccination with 1 × 104 TCID50 SUDV-Gulu. We assessed anaesthetised NHPs on days 28, 21, 14, and 7 before challenge; days 0, 3, 6, 9, 14, 21, 28, and 35 after challenge; and at euthanasia (day 40 for survivors). As we repurposed NHPs from a successful VSV-Ebola virus (EBOV) vaccine efficacy study, we also investigated VSV-EBOV’s cross-protective potential against SUDV challenge.
Findings
Of the six NHPs given VSV-SUDV, none showed any signs of disease in response to the challenge. Four of the five NHPs in the control group developed characteristic clinical signs of Sudan virus diseases. SUDV glycoprotein-specific IgG concentrations peaked 14 days after vaccination (titre of >1:10 000) and reached their highest concentrations at 6 days after challenge (1:25 600-1:102 400). Although the NHPs developed cross-reactive humoral responses to SUDV after VSV-EBOV vaccination and EBOV challenge, there was little cross-protection.
Interpretation
These data emphasise the need for species-specific vaccines for each human-pathogenic Ebolavirus. Furthermore, although previous VSV-EBOV immunity is boosted through VSV-SUDV vaccination, it only has a small effect on the immunogenicity and protective efficacy of VSV-SUDV vaccination against SUDV challenge.
Funding
Intramural Research Program, US National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Introduction
The 2022–23 Sudan virus (SUDV) outbreak in Uganda1 has focused public attention back on filoviruses, yet away from the well known Ebola virus (EBOV; member of the Zaire ebolavirus species). The outbreak started with a man aged 24 years who was diagnosed on Sept 19, 2022, after visiting several health clinics.1 Since Sept 19, 2022, cases have been reported from the Buyangabu, Kampala, Wasiko, Kagadi, Kyegegwa, Mubende, and Kassanda districts in central and west Uganda.2 On-site laboratory testing and medical support to identify and manage case patients is available in Uganda. Although licensed treatment and vaccines are currently not available to assist with outbreak management,1 countermeasures with preclinical efficacy were deployed to support Uganda’s outbreak response, including three vaccines and a monoclonal antibody treatment.3
Today six distinct species belonging to the genus Ebolavirus have been described,4 of which Zaire, Sudan, Bundibugyo, and Taï Forest ebolaviruses are known causes of human haemorrhagic disease.5 SUDV, the single virus member in the Sudan ebolavirus species, was codiscovered with EBOV in 1976 during an outbreak of viral haemorrhagic disease (now designated Sudan virus disease) in South Sudan. SUDV re-emerged in South Sudan in 1979 and 2004, causing smaller Sudan virus disease outbreaks. In 2000–01, SUDV emerged in Gulu, Uganda, causing the largest Sudan virus disease outbreak on record, with 425 cases and a case fatality rate of 53%.6 This emergence was followed by smaller outbreaks in Uganda in 2011, 2012, and 2012–13.7 The recent Sudan virus disease outbreak in Uganda accounted for a total of 162 cases (140 confirmed and 22 probable with 77 fatalities).8 The overall case fatality rate of SUDV infections is roughly 50% and the 2022–23 outbreak does not seem to differ substantially from previous outbreaks. Apart from outbreaks caused by SUDV, Uganda has previously reported outbreaks caused by Bundibugyo virus in 2007 and EBOV in 2019 (appendix p 5),7 which have average case fatality rates of 25% and 50%.9,10
There are currently two vaccines licensed for Ebola virus disease caused by EBOV, the single shot vesicular stomatitis virus [VSV]-EBOV vaccine (Ervebo, Merck, Kenilworth, NJ, USA) and the Ad26.ZEBOV/MVA-BN-Filo prime-boost approach (Zabdeno and Mvabea, Johnson & Johnson, New Brunswick, NJ, USA). Although VSV-EBOV would be immediately available, it is unlikely to cross-protect against SUDV infections due to antigenic differences of the viral glycoprotein.11 Ad26. ZEBOV/MVA-BN-Filo might be protective as the MVA-BN-Filo component includes an SUDV glycoprotein antigen; however, clinical data are not available. Multiple vaccine candidates targeting SUDV or several filoviruses (including SUDV) are in preclinical development.12 The vaccines are mainly based on platforms that have been investigated for EBOV-specific vaccines. These platforms include viral vectors (ie, human and chimpanzee adenoviruses, VSV, human parainfluenza virus-type 3, rabies virus, modified vaccinia Ankara [MVA], and Venezuelan equine encephalitis virus), virus-like particles, protein subunits, and DNA.12 Approaches with promising efficacy in preclinical non-human primate (NHP) studies are listed in the appendix (p 6). Only three candidate vaccines have been or are currently in phase 1a/b clinical trials addressing toxicity and immunogenicity: the chimpanzee adenovirus serotype 3 (cAd3) expressing the SUDV glycoprotein (NCT04041570 and NCT04723602), the chimpanzee adenovirus ChAdOx1-BiEBOV expressing the EBOV and SUDV glycoproteins (NCT0509750 and NCT05301504), and a DNA-based vaccine expressing Marburg virus (MARV), EBOV, and SUDV glycoproteins,13 which is no longer being pursued. Shortly after the Sudan virus disease outbreak in Uganda was confirmed, Merck donated a batch of bulk VSV-SUDV vaccine to Uganda to support outbreak management efforts.14 Thus, together with Ad26.ZEBOV/MVA-BN-Filo, there are four candidates to consider for potential use in the 2022–23 SUDV outbreak. In November, 2022, Ugandan authorities have agreed to vaccine trials of Sabin’s cAd3-SUDV (Sabin Vaccine Institute, Washington DC, USA), ChAdOx1-BiEBOV (Oxford University, Oxford, UK), and VSV-SUDV (Merck).15
In contrast to the VSV-SUDV vaccine expressing the SUDV-Boniface glycoprotein, we have developed a VSV-based SUDV-specific vaccine candidate expressing the SUDV-Gulu strain glycoprotein instead of the VSV glycoprotein (designated VSV-SUDV). SUDV-Gulu is the causative strain of the largest recorded Sudan virus disease outbreak, in 2000–01 in Uganda (appendix p 5) and phylogenetically closer to the 2022 outbreak strain than the original SUDV-Boniface strain. The vaccine vector is based on the VSV glycoprotein replacement approach and, thus, in principle, is identical to VSV-EBOV. Here, we investigated the protective efficacy of a single-dose vaccination with VSV-SUDV against SUDV challenge in the cynomolgus macaque model. Furthermore, we assessed the effect of pre-existing EBOV immunity on the protective efficacy of VSV-SUDV against SUDV challenge and decipher whether pre-existing VSV-EBOV immunity protects against SUDV challenge.
Methods
Ethics statement
All work involving EBOV and SUDV was done in the maximum containment laboratory at the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Rocky Mountain Laboratories, Hamilton, MT, USA. Rocky Mountain Laboratories is an Association for Assessment and Accreditation of Laboratory Animal Care-accredited institution. All procedures followed Rocky Mountain Laboratories Institutional Biosafety Committee-approved standard operating procedures. Animal work was done in strict accordance with the recommendations described by the Office of Animal Welfare and the Animal Welfare Act, US Department of Agriculture.16 This study was approved by the Rocky Mountain Laboratories Animal Care and Use Committee, and all procedures were on anesthetised animals by trained personnel under the supervision of board-certified clinical veterinarians. The NHPs were observed at least twice daily for clinical signs of disease according to a Rocky Mountain Laboratories Animal Care and Use Committee-approved scoring sheet and were humanely euthanised when they reached endpoint criteria.
NHPs were housed in adjoining individual primate cages that enabled social interactions under controlled conditions of humidity, temperature, and light (12 h light–dark cycles). Food and water were available ad libitum. NHPs were monitored and fed commercial monkey chow, treats, and fruit at least twice a day by trained personnel. Environmental enrichment consisted of commercial toys, music, video, and social interaction. All efforts were made to ameliorate animal welfare and minimise animal suffering in accordance with the Weatherall report17 on the use of NHPs in research.
Vaccine vectors
Previously described VSV-based vaccine vectors expressing the EBOV-Kikwit glycoprotein (VSV-EBOV)18 and VSV-MARV19,20 were used in this study. The VSV-SUDV was generated by cloning the SUDV-Gulu glycoprotein gene (NC_006432.1; version 8A) into the VSV backbone (appendix p 8), as previously described for other filovirus glycoproteins.21 Similarly, VSV-SUDV-GFP was generated by adding the GFP gene as an additional open reading frame between the SUDV glycoprotein and VSV-L genes (appendix p 8). We verified antigen expression by western blot analysis using anti-EBOV glycoprotein (ZGP 42/3.7, 1:10 000; provided by Ayato Takada, Hokkaido University, Sapporo, Japan), and anti-VSV-M (23H12, 1:1000; Kerafast, Boston, MA, USA).
Cells and challenge virus
Vero E6 cells (Mycoplasma negative; CVCL_0059) were grown at 37°C and 5% CO2 in Dulbecco’s modified Eagle’s medium (Sigma-Aldrich, St Louis, MO, USA) containing 10% fetal bovine serum (Wisent, St Bruno, QC, Canada), 2 mM L-glutamine, 50 U/mL penicillin, and 50 mg/mL streptomycin (all supplements from Thermo Fisher Scientific, Waltham, MA, USA). EBOV-Makona Guinea C07 was used as the challenge virus in the first study.18 SUDV-Gulu (NC_006432.1) was obtained from the US Army Medical Research Institute of Infectious Diseases. The virus was propagated once on Vero E6 cells, titred with median tissue culture infectious dose (TCID50) assay on Vero E6 cells and stored in liquid nitrogen. Deep sequencing revealed no contaminants; however, four base pair changes (three of them coding) were noted from this viral passage compared with its reference sequence (appendix p 7). We used a target dose of 10 000 TCID50 (back titred as 5623 TCID50) for the intramuscular SUDV challenge.
NHP study design
In this study, we used male or female cynomolgus macaques (n=11), aged 6–11 years and weighing 3·8–9·0 kg at the time of VSV-SUDV vaccination. The NHPs used in this study had been previously used in a published EBOV challenge study.18 In that study, nine animals were completely protected from disease after the EBOV challenge and never showed EBOV viraemia, and two animals developed mild disease with low level of EBOV viraemia.18 After the EBOV challenge, NHPs were rested for roughly 9 months before inclusion in this study. The NHPs were divided into two study groups, as outlined in the appendix (p 9). All animals received a 1 mL intramuscular injection for vaccination into two sites in the caudal thighs containing either 1 × 107 plaque-forming units (PFU) of VSV-SUDV (six NHPs) or 1 × 10⁷ PFU of VSV-MARV (control; five NHPs). 28 days after vaccination, all NHPs were challenged intramuscularly (day 0 after challenge) with 1 × 104 TCID50 SUDV-Gulu into two sites in the caudal thighs (appendix p 9). We assessed anaesthetised NHPs, including physical examination, rectal temperature measurement, bodyweight determination, and blood draws, on days 28, 21, 14, and 7 before challenge; days 0, 3, 6, 9, 14, 21, 28, and 35 after challenge; and at euthanasia (day 40 for survivors; humane endpoint for non-survivors) as outlined in the appendix (p 9). We used blood and serum samples to do complete blood cell counts, serum chemistry analysis, viraemia levels (appendix pp 2–3), and humoral immune response analysis by ELISA and neutralisation (appendix pp 3–4). Following euthanasia, we did a necropsy and collected samples of selected tissues including lymph nodes, liver, spleen, and adrenal gland for virological and histopathological analysis (appendix p 3).
Statistical analysis
We assessed differences in the survival curves using log-rank analysis. We compared humoral immune responses after vaccination and SUDV challenge at all timepoints between both groups by two-way ANOVA with Tukey’s multiple comparison test to evaluate statistical significance. Results with p<0·5 were considered statistically significant. Statistical analysis was done in Prism (version 9; GraphPad).
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
A total of 11 NHPs were vaccinated with VSV-SUDV or VSV-MARV approximately 1 year after an EBOV challenge study (appendix p 9). After 4 weeks, NHPs were challenged with a lethal dose of SUDV-Gulu and observed for 40 days. Vaccination with VSV-SUDV and VSV-MARV did not result in any obvious or noticeable adverse effects. After challenge on day 28 after vaccination, none of the VSV-SUDV-vaccinated macaques showed any signs of disease and were completely protected; four of five animals (80%) in the control group developed characteristic clinical signs of Sudan virus diseases, including petechial rash,22,23 and had to be euthanised according to our endpoint scoring criteria (figure 1A, B). Control NHPs succumbing to SUDV infection developed lymphocytopenia (figure 1C) and high-titre viraemia. However, despite developing lymphocytopenia, the one surviving control NHP was only weakly positive for SUDV RNA on day 6 after challenge and no virus isolation from whole blood was possible (figure 1D, E). Compared with the VSV-SUDV-vaccinated NHPs, the four control NHPs that succumbed to infection presented with increased aspartate aminotransferase, alkaline phosphatase, and blood urea nitrogen concentrations (figure 1F–H). In addition, the control NHPs that became infected had hypoalbuminaemia (figure 1I). All of these clinical chemistry changes are consistent with clinical Sudan virus diseases.22 The one surviving control NHP showed serum clinical chemistries similar to the protected VSV-SUDV-vaccinated NHPs.
Figure 1: Survival and clinical changes in NHPs after SUDV challenge.
Two groups of NHPs were vaccinated with VSV-SUDV (six NHPs) or control vaccine (VSV-MARV; five NHPs) and challenged 4 weeks later with SUDV. Survival (A), clinical scores (B), lymphocytes (C), and viraemia by real-time quantitative PCR (D) and titration (E) are shown. Serum concentrations of aspartate aminotransferase (F), alanine phosphatase (G), blood urea nitrogen (H), and albumin (I) were measured. Significant differences in the survival curves were determined using log-rank analysis. See appendix (pp 2–3) for details of TCID50 equivalents. MARV=Marburg virus. NHP=non-human primate. SUDV=Sudan virus. TCID50=median tissue culture infectious dose. VSV=vesicular stomatitis virus.
At the time of euthanasia, the control macaques presented with liver and spleen pathology as previously described for SUDV infections.22 Histologically, the control NHPs had liver lesions characteristic of Sudan virus diseases, including multifocal to coalescing hepatocellular degeneration and necrosis with acute inflammation and abundant microfibrin thrombi (figure 2A). In the spleen white pulp, we observed necrosis and loss with abundant fibrin effacing the red pulp. Immunohistochemical evaluation showed abundant viral antigen associated with these hepatic and splenic lesions (figure 2A). High SUDV titres were found in target tissues, such as liver, spleen, adrenal glands, lymphoid tissues, urinary bladder, and muscle at the injection site (figure 2B). We performed necropsies of vaccinated NHPs and of the one survivor in the control group on day 40 after challenge. All collected tissue samples were essentially normal with no evidence of immunoreactivity in liver or spleen (figure 2A).
Figure 2: Histopathology after SUDV challenge.

(A) At the time of euthanasia, liver and spleen necropsy samples were collected (day 6–7 after challenge for the control group; 40 days after challenge for the vaccinated group), inactivated, processed, and stained with haematoxylin and eosin (upper row of images). Immunohistochemistry of samples is shown in the bottom row of images. SUDV antigen (VP40) was detected in control NHPs only. Immunoreactive cells are brown. Sections from a representative animal in each group are shown. Magnification ×200; scale bar 50 μm. (B) SUDV titres in control NHPs at the time of euthanasia. NHP=non-human primate. SUDV=Sudan virus. TCID50=median tissue culture infectious dose.
We focused on the humoral immune response as peripheral blood mononuclear cells for T-cell responses were not collected in this study. Before VSV-SUDV or VSV-MARV vaccination, all animals showed an EBOV glycoprotein-specific IgG titre of more than 1:1000 (appendix p 9). This response was significantly boosted with the VSV-SUDV vaccination by more than an order of magnitude. By contrast, no boosting effect was noticed with the VSV-MARV vaccination (figure 3A). The SUDV challenge did not boost the EBOV glycoprotein-specific IgG titres in any of the NHPs except for in the single survivor in the control group, which showed a steep booster effect peaking at 14 days after challenge, indicative of an anamnestic response (figure 3A).
Figure 3: Humoral immune responses after vaccination and SUDV challenge.
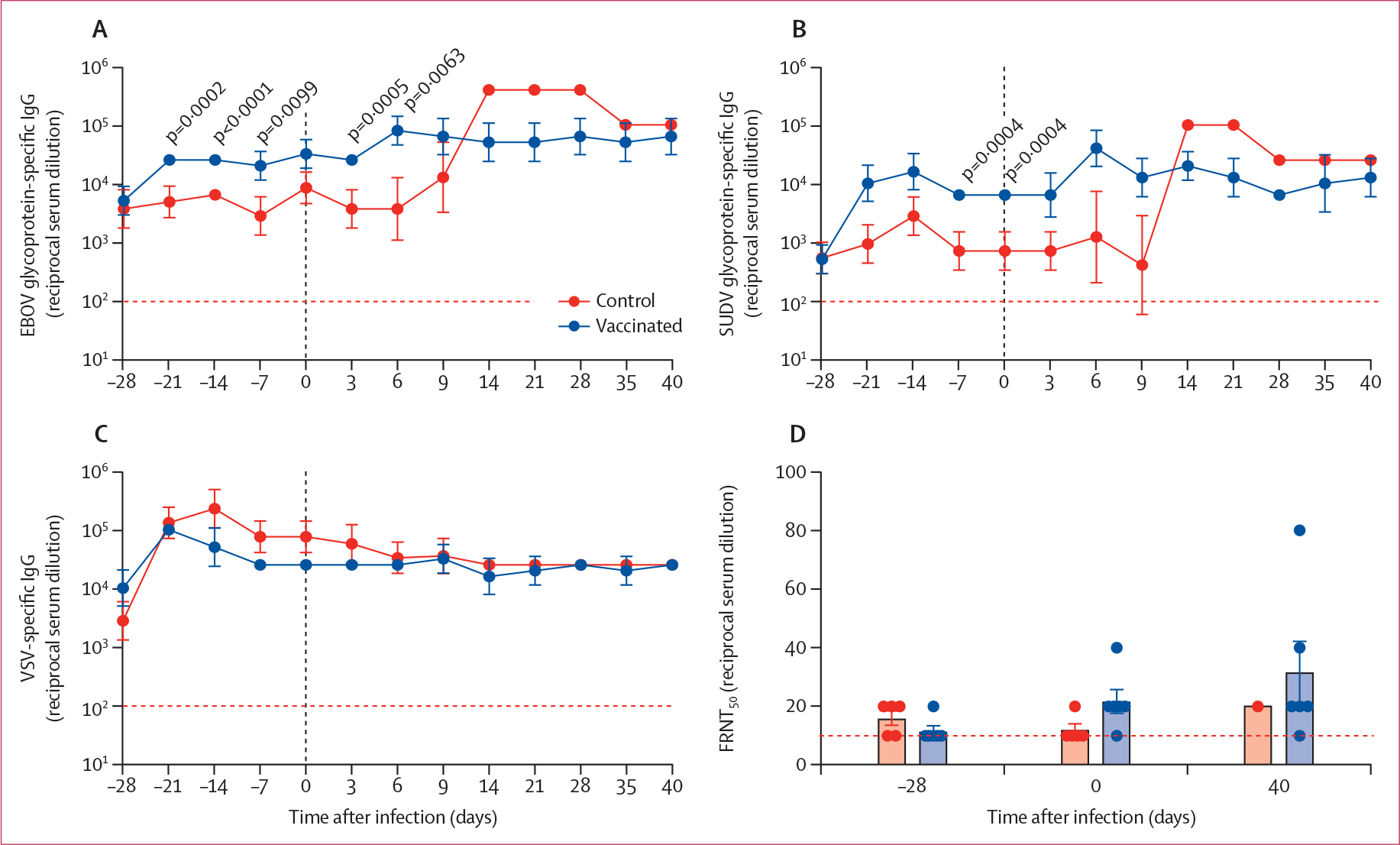
Serum IgG concentrations specific for EBOV glycoprotein (A), SUDV glycoprotein (B), and VSV (C) were measured over time. Geometric mean and geometric SD are shown. Statistical significance was determined by two-way ANOVA with Tukey’s multiple comparisons test. (D) Serum neutralisation presented as FRNT50 of green fluorescence protein-positive cells at the time of vaccination (day 28 before challenge), challenge (day 0 after challenge), and euthanasia (day 40 after challenge [study end]). The dotted line shows the limit of detection. EBOV=Ebola virus. FRNT50=50% fluorescence reduction. SUDV=Sudan virus. VSV=vesicular stomatitis virus.
Investigation of the SUDV glycoprotein-specific IgG response revealed that all macaques showed some cross-reactive antibodies from the previous VSV-EBOV vaccination and EBOV challenge before VSV-SUDV and VSV-MARV vaccination (figure 3B; appendix p 9). The SUDV glycoprotein-specific IgG concentration peaked 14 days after vaccination (titre of >1:10 000) and was slightly boosted by the SUDV infection, reaching its highest titre at day 6 after challenge (1:25 600–1:102 400; figure 3B). The sole survivor among the control animals showed a steep booster effect after SUDV challenge peaking at day 14 after challenge, which is indicative of an anamnestic response (figure 3B).
We compared the VSV-specific IgG over the course of the experiment (68 days), and we observed peak titres 1–2 weeks after vaccination without a significant difference between the VSV-SUDV study group and VSV-MARV control group (figure 3C). These titres stabilised by day 0 after challenge and remained constant throughout the study.
Analysis of neutralising immune responses using a VSV-SUDV-GFP-based assay revealed low neutralising activity in all vaccinated NHPs at the time of challenge and at study end (figure 3D). Remarkably, the serum of the single surviving control NHP showed only a small increase in neutralising activity on 40 days after challenge despite the strong increase of SUDV glycoprotein-specific IgG responses after challenge.
Discussion
Here, we show that, in the cynomolgus macaque model, a single-dose vaccination with VSV-SUDV is uniformly protective against SUDV challenge. We also show that pre-existing EBOV immunity does not affect the protective efficacy of VSV-SUDV against SUDV challenge, and that pre-existing VSV-EBOV immunity does not protect against SUDV challenge despite cross-reactive immune responses.
The recent Sudan virus disease outbreak in Uganda has shown that the public health response to emerging or re-emerging infectious diseases lacks accredited and stockpiled vaccine and treatment options. Despite great effort and success in treating EBOV, there still is a general absence of available countermeasures against infections with other human-pathogenic filoviruses.24 Disappointingly, the situation with vaccines and treatment options against Ebolaviruses in general has barely changed since the EBOV epidemic in west Africa more than 8 years ago. Multiple SUDV vaccine candidates (appendix p 6) and some treatment options have finished preclinical evaluation and are awaiting clinical trials, yet nothing was immediately ready for deployment when the Sudan virus disease outbreak was declared in September, 2022.
Clinically, Sudan virus disease is indistinguishable from Ebola virus disease and Marburg virus disease;5 however, the pathogens are genetically and antigenically distinct enough to render specific vaccines and monoclonal antibody treatments ineffective against a heterologous infection.25 Hence, EBOV-specific vaccines, such as VSV-EBOV, are not expected to be effective against SUDV infections due to the absence of cross-protective immune responses, as demonstrated in this study. Therefore, we generated a VSV-SUDV vector by simply exchanging the EBOV glycoprotein with the SUDV glycoprotein to be used as a single-dose, live-attenuated vaccine against Sudan virus disease. We demonstrated that VSV-SUDV protected cynomolgus macaques against lethal challenge with SUDV. Vaccinated animals did not develop viraemia, organ tissue viral loads or damage, or clinical disease, which is in strong contrast to the VSV-MARV-vaccinated control NHPs. Interestingly, one control-vaccinated NHP survived with a clinical progression similar to the vaccinated NHPs. This might be explained by the published observation that the cynomolgus macaque model for Sudan virus disease is not uniformly lethal.26 However, the almost complete absence of Sudan virus disease parameters in this surviving animal is surprising and might indicate some cross-protective immune responses in this animal. This possibility is supported by a strong anamnestic IgG response to SUDV challenge. Future studies challenging VSV-EBOV-vaccinated NHPs with SUDV should support or disprove this finding.
This unique study design allowed us to decipher potential cross-reactive or cross-protective immune responses between EBOV and SUDV. All macaques had detectable EBOV glycoprotein-specific IgG antibody titres, approximately 1 year after VSV-EBOV vaccination and EBOV challenge (appendix p 9). Macaques also maintained similar levels of VSV-specific immune responses before start of the VSV-SUDV vaccine study. Neither EBOV nor VSV pre-existing immunity hampered the development of SUDV glycoprotein-specific humoral immune responses, highlighting the reusability of VSV-ΔG vectors with heterologous glycoproteins (as previously shown27). By contrast, the VSV-SUDV vaccination boosted the EBOV glycoprotein-specific responses, probably adding a durability benefit of these protective responses. The SUDV glycoprotein-specific immune responses were protective against SUDV challenge, whereas the EBOV glycoprotein-specific immune responses were not. Therefore, cross-reactive antibodies for EBOV and SUDV were generated by macaques; however, these antibodies were unlikely to cross-protect against heterologous challenge. Although not investigated in this study, it appears that the induced T-cell responses to VSV vaccination and EBOV or SUDV challenge cannot overcome the absence of cross-protective antibodies, which might also indicate a minor role of T-cell responses to the overall efficacy of VSV-ΔG vectored vaccines.28
The study has limitations that will be addressed by our future work. A VSV-EBOV-vaccinated group would have been an important control to address the role of cross-protection. However, the study was initially designed to investigate the protective efficacy of VSV-SUDV against SUDV challenge, and VSV-MARV vaccination was chosen as control vaccine to limit cross-protective properties. The absence of T-cell analysis is a limitation as a complete evaluation of immune responses should include T-cell immune responses, although immune response analyses of VSV-vectored filovirus vaccines have indicated a minor role of T cells.28 Furthermore, the current study does not address the fast-acting efficacy of VSV-SUDV, which would have been beneficial for the potential use of this vaccine in ring vaccination strategies for outbreak response. However, the VSV-SUDV vaccine provided 100% protection of macaques when it was administered as a post-exposure treatment shortly after challenge,29 suggesting possible rapid protection from VSV-SUDV vaccines, similar to the protection from VSV-EBOV.30,31
In conclusion, VSV-SUDV completely protects macaques against lethal SUDV challenge and probably acts similarly to other VSV-ΔG-based filovirus vaccines, including VSV-EBOV and VSV-MARV. The vaccine is a potent candidate for clinical trials, including for ring vaccination for which this vaccine was developed. Similar to VSV-EBOV, the replicating nature of VSV-SUDV is likely to confer a rapid onset of protective immunity. The absence of cross-protection from VSV-EBOV vaccination and EBOV challenge supports the ineffectiveness of the licensed VSV-EBOV in the 2022–23 SUDV outbreak. However, the boost of the EBOV glycoprotein-specific response through VSV-SUDV vaccination might add a benefit to the durability of protection against Ebola virus disease, which needs to be further assessed. Finally, VSV-SUDV is intended as a vaccine in individuals, such as front-line workers, regardless of previous VSV-EBOV vaccination status.
Supplementary Material
Research in context.
Evidence before this study
We searched PubMed for articles describing Sudan virus (SUDV)-specific vaccination approaches with protective efficacy in non-human primate (NHP) models published between Jan 1, 2000, and Oct 10, 2022, with no language restrictions using the medical subject headings search terms “Sudan virus”, “Sudan ebolavirus”, “nonhuman primate” and “vaccine”. We identified several studies with protective efficacy against SUDV. We did not find any study describing prophylactic vesicular stomatitis virus (VSV)-based SUDV vaccination that conferred uniform protection using the VSV-ΔG vector in NHPs. This emphasised the need to conduct studies evaluating the protective efficacy of VSV-SUDV in NHPs naive to SUDV infection or in the context of pre-existing VSV-Ebola virus (EBOV) immunity.
Added value of this study
We show that a single high dose of VSV-SUDV protects NHPs from lethal disease within 4 weeks, and that pre-existing immunity to VSV-EBOV does not affect VSV-SUDV immunogenicity and protective efficacy. In addition, we demonstrate that VSV-EBOV vaccination 1 year before SUDV infection does not provide cross-protection against Sudan virus disease.
Implications of all the available evidence
Our results initially demonstrate the protective efficacy of the VSV-SUDV vaccine in the NHP model. The results suggest that VSV-EBOV does not provide sufficient cross-protection against Sudan virus disease, highlighting the need for species-specific vaccine development against filoviruses. We report a boosting effect of VSV-SUDV vaccination on VSV-EBOV immunity that is likely to add a durability benefit for protective responses against Ebola virus disease. Finally, our data provide strong support for the use and re-use of the VSV vaccine platform for ring vaccination during outbreaks of emerging infectious diseases including Sudan virus disease.
Acknowledgments
We thank members of the Rocky Mountain Veterinary Branch, US National Institute of Allergy and Infectious Diseases (NIAID), for supporting the non-human primate study. We are grateful to the US Army Medical Research Institute of Infectious Diseases for sharing their virus isolate. The study was funded by the Intramural Research Program, NIAID, US National Institutes of Health.
Footnotes
Declaration of interests
HF claims intellectual property of VSV-based filovirus vaccines. All other authors declare no competing interests.
Contributor Information
Andrea Marzi, Laboratory of Virology, Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Rocky Mountain Laboratories, Hamilton, MT, USA.
Paige Fletcher, Laboratory of Virology, Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Rocky Mountain Laboratories, Hamilton, MT, USA.
Friederike Feldmann, Rocky Mountain Veterinary Branch, Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Rocky Mountain Laboratories, Hamilton, MT, USA.
Greg Saturday, Rocky Mountain Veterinary Branch, Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Rocky Mountain Laboratories, Hamilton, MT, USA.
Patrick W Hanley, Rocky Mountain Veterinary Branch, Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Rocky Mountain Laboratories, Hamilton, MT, USA.
Heinz Feldmann, Laboratory of Virology, Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Rocky Mountain Laboratories, Hamilton, MT, USA.
Data sharing
All data is presented in the paper and available from the corresponding author on request.
References
- 1.WHO. Ebola disease caused by Sudan virus—Uganda. Sept 26, 2022. https://www.who.int/emergencies/disease-outbreak-news/item/2022-DON410 (accessed Dec 3, 2022).
- 2.WHO. Ebola virus disease in Uganda SitRep—93. https://www.afro.who.int/countries/uganda/publication/ebola-virus-disease-uganda-sitrep-93 (accessed Oct 27, 2022).
- 3.Steenhuysen J US sends experimental antibody, antiviral drug to Uganda for Ebola outbreak. Oct 18, 2022. https://www.reuters.com/business/healthcare-pharmaceuticals/us-sends-experimental-antibody-antiviral-drug-uganda-ebola-outbreak-2022-10-18/ (accessed Dec 3, 2022).
- 4.Kuhn JH, Amarasinghe GK, Basler CF, et al. ICTV virus taxonomy profile: filoviridae. J Gen Virol 2019; 100: 911–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jacob ST, Crozier I, Fischer WA 2nd, et al. Ebola virus disease. Nat Rev Dis Primers 2020; 6: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Okware SI, Omaswa FG, Zaramba S, et al. An outbreak of Ebola in Uganda. Trop Med Int Health 2002; 7: 1068–75. [DOI] [PubMed] [Google Scholar]
- 7.US Centers for Disease Control and Prevention. Ebola (Ebola virus disease)—outbreaks. 2022. https://www.cdc.gov/vhf/ebola/history/chronology.html (accessed Oct 10, 2022).
- 8.Medecins Sans Frontieres. One month after declaration of Ebola epidemic in Uganda. Oct 25, 2022. https://www.msf.org/one-month-after-declaration-ebola-epidemic-uganda (accessed Oct 25, 2022).
- 9.WHO. Ebola virus disease. Feb 23, 2021. https://www.who.int/news-room/fact-sheets/detail/ebola-virus-disease (accessed Oct 10, 2022).
- 10.Kadanali A, Karagoz G. An overview of Ebola virus disease. North Clin Istanb 2015; 2: 81–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Geisbert TW, Geisbert JB, Leung A, et al. Single-injection vaccine protects nonhuman primates against infection with Marburg virus and three species of Ebola virus. J Virol 2009; 83: 7296–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.WHO. Sudan Ebolavirus vaccine tracker—list of vaccine candidates in research & development. Sept 26, 2022. https://www.who.int/publications/m/item/sudan-virus-vaccine-tracker---list-of-vaccine-candidates-in-research---development (accessed Oct 10, 2022).
- 13.Sarwar UN, Costner P, Enama ME, et al. Safety and immunogenicity of DNA vaccines encoding Ebolavirus and Marburgvirus wild-type glycoproteins in a phase I clinical trial. J Infect Dis 2015; 211: 549–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cohen J Forgotten Ebola vaccine could help in outbreak. Science 2022; 378: 340–41. [DOI] [PubMed] [Google Scholar]
- 15.Branswell H WHO, Uganda plan to test three candidate Ebola vaccines in outbreak. Nov 16, 2022. https://www.statnews.com/2022/11/16/who-uganda-plan-to-test-three-candidate-ebola-vaccines-in-outbreak/ (accessed Dec 3, 2022).
- 16.US National Research Council of the National Academies. Guide for the care and use of laboratory animals. https://grants.nih.gov/grants/olaw/guide-for-the-care-and-use-of-laboratory-animals.pdf (accessed Jan 23, 2023).
- 17.The Royal Society. The Weatherall report on the use of non-human primates in research. Dec 12, 2006. https://royalsociety.org/policy/publications/2006/weatherall-report/ (accessed Dec 3, 2022).
- 18.Marzi A, Robertson SJ, Haddock E, et al. Ebola Vaccine. VSV-EBOV rapidly protects macaques against infection with the 2014/15 Ebola virus outbreak strain. Science 2015; 349: 739–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marzi A, Menicucci AR, Engelmann F, et al. Protection against Marburg virus using a recombinant VSV-vaccine depends on T and B cell activation. Front Immunol 2019; 9: 3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marzi A, Jankeel A, Menicucci AR, et al. Single dose of a VSV-based vaccine rapidly protects macaques from Marburg virus disease. Front Immunol 2021; 12: 774026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marzi A, Ebihara H, Callison J, et al. Vesicular stomatitis virus-based Ebola vaccines with improved cross-protective efficacy. J Infect Dis 2011; 204 (suppl 3): S1066–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Woolsey C, Fears AC, Borisevich V, et al. Natural history of Sudan Ebolavirus infection in rhesus and cynomolgus macaques. Emerg Microbes Infect 2022; 11: 1635–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carbonnelle C, Moroso M, Pannetier D, et al. Natural history of Sudan Ebolavirus to support medical countermeasure development. Vaccines (Basel) 2022; 10: 963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cross RW, Longini IM, Becker S, et al. An introduction to the Marburg virus vaccine consortium, MARVAC. PLoS Pathog 2022; 18: e1010805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hargreaves A, Brady C, Mellors J, Tipton T, Carroll MW, Longet S. Filovirus neutralising antibodies: mechanisms of action and therapeutic application. Pathogens 2021; 10: 1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bennett RS, Huzella LM, Jahrling PB, Bollinger L, Olinger GG Jr, Hensley LE. Nonhuman primate models of Ebola virus disease. Curr Top Microbiol Immunol 2017; 411: 171–93. [DOI] [PubMed] [Google Scholar]
- 27.Marzi A, Feldmann F, Geisbert TW, Feldmann H, Safronetz D. Vesicular stomatitis virus-based vaccines against Lassa and Ebola viruses. Emerg Infect Dis 2015; 21: 305–07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Menicucci AR, Sureshchandra S, Marzi A, Feldmann H, Messaoudi I. Transcriptomic analysis reveals a previously unknown role for CD8+ T-cells in rVSV-EBOV mediated protection. Sci Rep 2017; 7: 919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Geisbert TW, Daddario-DiCaprio KM, Williams KJ, et al. Recombinant vesicular stomatitis virus vector mediates postexposure protection against Sudan Ebola hemorrhagic fever in nonhuman primates. J Virol 2008; 82: 5664–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Feldmann H, Jones SM, Daddario-DiCaprio KM, et al. Effective post-exposure treatment of Ebola infection. PLoS Pathog 2007; 3: e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marzi A, Hanley PW, Haddock E, Martellaro C, Kobinger G, Feldmann H. Efficacy of vesicular stomatitis virus-Ebola virus postexposure treatment in rhesus macaques infected with Ebola virus makona. J Infect Dis 2016; 214 (suppl 3): S360–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data is presented in the paper and available from the corresponding author on request.


