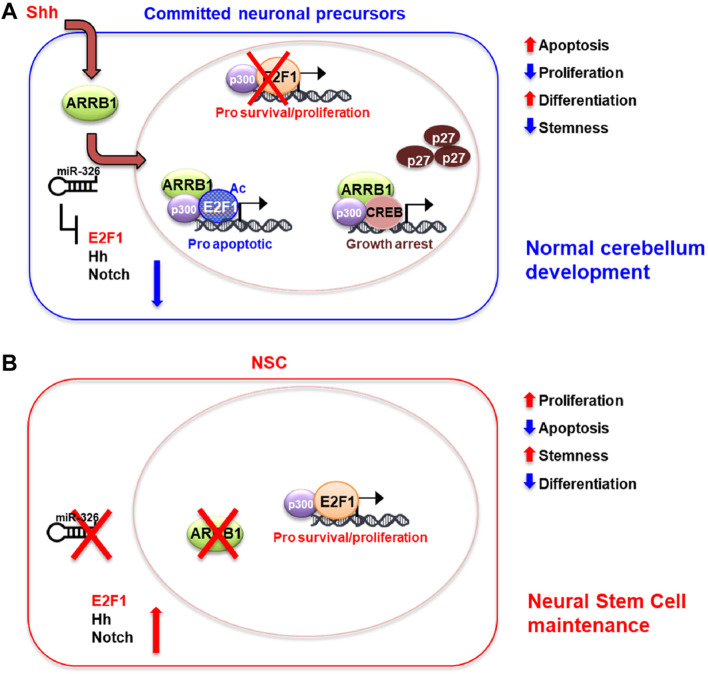
FIGURE 5.
Schematic model of ARRB1/E2F1-ac functions in GCPs and NSCs. Overview of roles played by ARRB1/E2F1-ac in normal cerebellar development. (A): Committed neuronal precursors (i.e., NSCs grown in DFM, GCPs). In our previous works, we identified miR-326 as a miRNA necessary for maturation of granule cell progenitors (GCPs) into mature granule cells (Ferretti et al., 2008). Moreover, this miRNA is integrated into the first intron of the Arrb1 gene and shares the same regulatory regions as its host gene. miR-326 also contributes to ARRB1 functions by blunting proliferative signals mediated by E2F1, Hedgehog, and Notch, and by promoting cell differentiation (Ferretti et al., 2008; Kefas et al., 2009; Po et al., 2017; Miele et al., 2021). Committed neuronal precursors express ARRB1 and mir-326, which regulate their development at multiple levels. Shh signaling upregulates ARRB1 levels and promotes its translocation to the nucleus. There ARRB1, in complex with P300, induces acetylation of E2F1 (E2F1-ac), redirecting the transcription factors activity from survival/proliferative gene targets towards those that promote apoptosis (Trp73, Caspases 3 and 7). Interacting with CREB and P300, ARRB1 upregulates the expression and nuclear accumulation of P27, which eventually blocks cell cycle progression. miR-326 favors neuronal cell differentiations by inhibiting multiple survival/proliferative signaling: E2F1, Hedgehog (Hh) and Notch via direct binding of the 3′-UTRs of E2f1, Smo, Gli2, Notch1 and Notch2. (B): In NSCs, non-expression of ARRB1 and miR-326 promotes cell proliferation, survival, and stemness by favoring non-acetylated E2F1 activity and active Hedgehog (Hh) and Notch signaling.