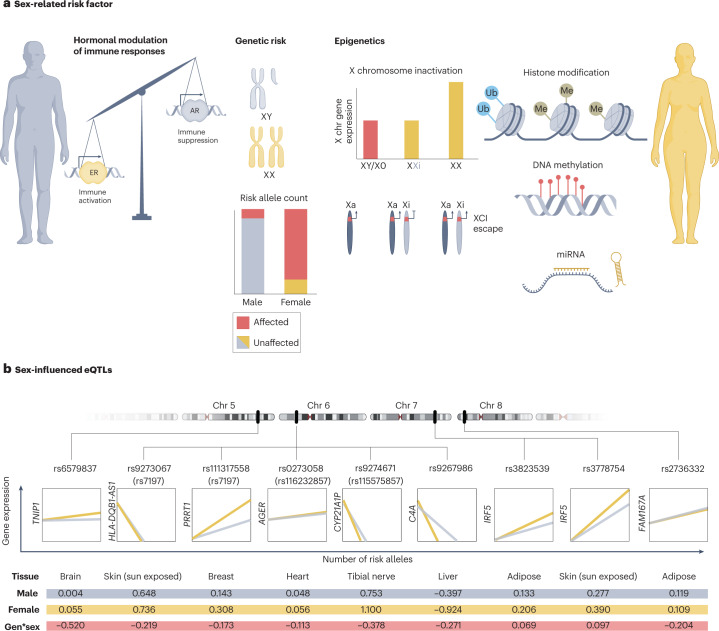
Fig. 5. Potential mechanisms connecting biological sex with risk of primary Sjögren syndrome.
Sex hormones affect biological functions directly and indirectly. The figure illustrates how genetic risk factors can result in discordant effects depending on the sex of the individual. a, Suggested hypotheses to explain this discordance include differential hormonal modulation of immune responses, men requiring a higher number of genetic risk loci to develop disease and differences in X chromosome (X chr) dosage. Genes located on the X chromosome, including important immune genes such as TLR7, can escape X chromosome inactivation (XCI), resulting in higher expression of the protein in women than in men. Other epigenetic modifications can also differ between men and women, including microRNA (miRNA) expression, histone modification and DNA methylation patterns. b, Some single nucleotide polymorphisms (SNPs) associated with primary Sjögren syndrome (pSS) affect the expression of genes in a sex-dependent fashion (known as sex-influenced expression quantitative trait loci (eQTL)). Variants associated with pSS with discordant effects on gene expression between men and women that reach a particular significance threshold (P < 0.05) for sex–genotype interactions are included (data taken from the Genotype-Tissue Expression (GTEx) database). All variants in high linkage disequilibrium with the Sjögren syndrome-associated variants were considered. Variants reported as associated with pSS (Table 1) are included in parenthesis if a sex-eQTL effect was identified for a linked variant. The table summarizes the tissue the effect was identified in, the effect size (expressed as a regression coefficient) in men and in women, and the genotype-by-sex (gen*sex) interaction effect size. AR, androgen receptor; ER, oestrogen receptor; Me, methyl group; Ub, ubiquitin.