Keywords: atherosclerosis, health promotion, metabolism, pediatrics, type 2 diabetes
Abstract
In pediatric population with diabetes and obesity, insulin resistance (HOMA-IR) has been associated with worsening vascular outcomes, however, the cumulative role of HOMA-IR, hyperglycemia, and hyperinsulinemia on repeatedly measured vascular outcomes in asymptomatic youth is unknown. We examined the longitudinal associations of fasting glucose, insulin, and HOMA-IR with carotid-femoral pulse wave velocity (cfPWV) and carotid intima-media thickness (cIMT). From the Avon Longitudinal Study of Parents and Children (ALSPAC) birth cohort, UK 1,779, 15-yr-old participants were followed up for 9 yr. Glucose, insulin, and HOMA-IR assessed at 15, 17, and 24 yr and sex-specifically dichotomized as ≥75th percentile, indicating high category and <75th percentile as reference. cfPWV and cIMT were measured at ages 17 and 24 yr. Associations were examined using linear mixed-effect models adjusted for cardiometabolic and lifestyle covariates. Among 1,779 participants [49.9% female], glucose, insulin, and HOMA-IR had a J- or U-shaped increase from ages 15 through 24 yr. The cumulative exposures to hyperinsulinemia effect estimate −0.019 mU/L; [95% CI −0.019 to −0.002; P = 0.033] and high HOMA-IR: −0.021; [−0.039 to −0.004; P = 0.019] from 15 to 24 yr of age were negatively associated with the 7-yr cfPWV progression. Only cumulative hyperinsulinemia and high HOMA-IR from ages 15 to 17 yr but not from ages 17 to 24 yr was associated with decreased cfPWV progression. There were no associations between cumulative hyperglycemia and cfPWV or cIMT progression. Hyperinsulinemia and HOMA-IR were not associated with cIMT progression. In conclusion, late adolescence may be an optimal timing for intervention targeted at sustaining the protective effect of the decline of insulin and insulin resistance on arterial stiffness progression.
NEW & NOTEWORTHY Fasting plasma glucose, insulin, and insulin resistance had a J- or U-shaped increase from 15 to 24 yr with the base of the curve at age 17 yr. Cumulative high insulin and high insulin resistance from 15 to 24 yr were negatively associated with arterial stiffness progression from ages 17 to 24 yr. Age 17 yr may be an optimal timing for intervention targeted at sustaining the protective effect of the decline of insulin and insulin resistance on arterial stiffness progression.
INTRODUCTION
Arterial stiffness, measured as carotid-femoral pulse wave velocity (cfPWV), and carotid intima-media thickness (cIMT) are well-established risk factors for cardiovascular morbidity and mortality across the life course (1–5). Metabolic derangements and altered metabolic indices such as dysglycemia, hyperinsulinemia, and insulin resistance, have been identified as precursors of poor arterial health among adults (6–8). A longitudinal study among adolescents with type 2 diabetes mellitus reported worsening arterial stiffness (9). We have recently shown that arterial stiffness in late adolescence may be causally associated with insulin resistance and hyperinsulinemia in young adulthood (4, 5). Cross-sectional studies among moderate sample-sized healthy adolescents concluded that insulin resistance was not associated with large artery stiffness (10, 11). Nonetheless, there is a paucity of large prospective studies among healthy adolescents and young adults, which would clarify the independent roles of metabolic indices on arterial health, particularly during growth and maturation in mid-adolescence across sexes.
From a public health and clinical perspective, it is important to determine the age at which metabolic alterations lead to poor arterial health in the young population and an appropriate time for an intervention. Therefore, we examined the longitudinal associations of repeated measures of fasting glucose, insulin, and insulin resistance at ages 15, 17, and 24 yr with cfPWV and cIMT progression from ages 17 through 24 yr using data from the Avon Longitudinal Study of Parents and Children (ALSPAC) birth cohort, England, United Kingdom. We hypothesized that cumulative fasting glucose, insulin, and insulin resistance linearly increase from 15 through 24 yr and this increase would be positively associated with worsening arterial stiffness and cIMT progression.
METHODS
Study Cohort
Data were from the ALSPAC birth cohort, which investigates factors that influence childhood development and growth. Altogether, 14,541 pregnant women from Avon, southwestern England, UK, who had a total of 14,676 fetuses, were enrolled between April 1, 1991, and December 31, 1992. When the oldest children were ∼7 yr of age, an attempt was made to bolster the initial sample size with eligible cases who had failed to join the study originally resulting in 14,901 children alive at 1 yr of age. Regular clinic visits of the children commenced at 7 yr of age and are still ongoing into adulthood. Study data at 24 yr of age were collected and managed using REDCap electronic data capture tools (12). In the prospective analyses, 1,779 participants who had complete clinic measurements for fasting insulin and glucose at 15 and 17 yr and cfPWV measure at 17 yr during follow-up clinic visits were eligible for analyses (Supplemental Fig. S1). The excluded participants who had only fasting samples 17 yr of age were similar to those included in the study (Supplemental Table S1). Ethical approval for the study was obtained from the ALSPAC Ethics and Law Committee and the Local Research Ethics Committees. Informed consent for the use of data collected via questionnaires and clinics was obtained from participants following the recommendations of the ALSPAC Ethics and Law Committee at the time (13–15). Consent for biological samples has been collected in accordance with the Human Tissue Act (2004). Please note that the study website contains details of all the data that is available through a fully searchable data dictionary and variable search tool (http://www.bristol.ac.uk/alspac/researchers/our-data/).
Anthropometry and Body Composition
Anthropometry (height and weight) of participants at age 15, 17, and 24 yr was assessed observing standard protocols and body mass index (BMI) was computed as weight in kilograms per height in meters squared (1). Body composition (total fat mass, and lean mass) was assessed using a dual-energy X-ray absorptiometry scanner at 15, 17, and 24 yr as previously described (1, 16). Time (years) to age at peak height velocity, an objective measure of pubertal or maturation status without having to rely on physical examination or self-report, was derived using Superimposition by Translation And Rotation mixed-effects growth curve analysis (17).
Vascular Measures
At age 17 and 24 yr, cfPWV was computed from pressure waveforms obtained using the Vicorder device (Skidmore Medical, Bristol, UK) observing standard protocols as detailed earlier (1, 4). A cuff was placed over the right carotid artery in the participant’s neck, whereas another was located over the femoral artery in their upper right thigh. The distance between the participant’s suprasternal notch and the top of the thigh cuff was measured, as was the distance between their suprasternal notch and the bottom of the neck cuff on the right side. cfPWV and transit time to the nearest 0.01 ms were automatically computed from measurements of pulse transit time and distance traveled by the pulse between two recording sites using Vicorder (Skidmore Medical, Bristol, UK) portable physiologic vascular testing equipment. All measurements were taken independently by one of two trained vascular technicians (interobserver mean difference 0·2 m/s, SD 0·1). cfPWV was recorded three times and average was computed for analysis.
cIMT from the right and left common carotid arteries at 17 yr was assessed by ultrasound using a linear 12-MHz transducer (Vivid7, GE Medical, Chicago, IL), and cIMT from the right and left common carotid arteries at 24 yr was measured using an ultrasound machine (CardioHealth Panasonic and a 13.5 MHz linear array broadband transducer (probe; center frequency 9.0 MHz) (1, 4). All vascular measures at 17 and 24 yr were extensive and rigorous as earlier described, interobserver variability for cIMT was assessed in a separate sample of 25 young adults (coefficient of variation: 4.4 ± 2.2%) (1, 4). Participants were placed in a supine position with the head rotated by 45 degrees from the midpoint. An automated guide line was placed at the bulb (a longitudinal scan that included the common carotid artery and the carotid bifurcation) with the region-of-interest box and IMT trace lines automatically positioned 1 cm away from the guide line. The scanner automatically saved an image when the region-of-interest box turned green, indicating good image quality. An automated cIMT measurement, recorded from the posterior wall of the artery, was saved after three consecutive cardiac cycles. When interrogating the common carotid, the CardioHealth system calculated and displayed the cIMT that is updated at each detected R-wave of the cardiac cycle. Once the measurement achieved a predefined quality threshold, scanning automatically stopped and a report was generated. Raw data were checked for outliers and cIMT value >1.0 mm was reviewed by a trained research scientist to assess validity. Abnormal values due to measurement error were removed. Participants had between 1 to 3 cIMT measures for each of the right and left carotid arteries. For our analysis, we computed the mean of the average measurement of the right and left common carotid arteries as cIMT (1, 4).
Cardiometabolic and Lifestyle Factors
Heart rate and blood pressure were measured at ages 15, 17, and 24 yr as previously detailed (1). Participants at age 17 and 24 yr were classified as hypertensive if systolic and diastolic blood pressure is ≥140/90 mmHg, while at age 15 yr if systolic and diastolic blood pressure is ≥95th percentile (18, 19). Using standard protocols, blood samples at ages 15, 17, and 24 yr were collected, spun, and frozen at –80°C and a detailed assessment of fasting glucose, insulin, high-sensitivity C-reactive protein, low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol, and triglycerides, has been reported (coefficient of variation was <5%) (1, 16, 20). Fasting insulin was measured using an ultrasensitive automated microparticle enzyme immunoassay (Mercodia), which does not cross-react with proinsulin (21) and the sensitivity of the immunoassay was 0.07 mU/L. We calculated homeostatic model assessment of insulin resistance (HOMA-IR) from (fasting plasma insulin x fasting plasma glucose/22.5) (22). Categories of fasting glucose, insulin, and HOMA-IR binary categories were grouped as ≥75 percentile as high and <75 percentile as moderate, normal, healthy, or not high. The normal or healthy group was the reference category. This percentile cutpoint corresponds to concentration of glucose >6.1 mmol/L and insulin >11.78 mU/L (23).
Questionnaires to assess smoking behavior were administered at the 15-, 17-, and 24-yr clinic visits (1). At the 17-yr clinic visit, participants were briefly asked about their personal and family (mother, father, and siblings) medical history such as a history of hypertension, diabetes, high cholesterol, and vascular disease (1). Sedentary time, light physical activity, and moderate-to-vigorous physical activity at age 15 yr were assessed with ActiGraphTM accelerometer worn for 7 days. At 24 yr, sedentary time, light physical activity, and moderate-to-vigorous physical activity were assessed using ActiGraph GT3X+ accelerometer device worn for four consecutive days, ideally starting the day after the clinic visit (1).
Statistical Analysis
Cohort descriptive characteristics were summarized as means and standard deviation, medians, and interquartile ranges, or frequencies and percentages. We explored sex differences using independent t tests, Mann–Whitney U tests, or chi-square tests for normally distributed, skewed or dichotomous variables, respectively. Normal distribution of variables was assessed by histogram curve, quantile-quantile plot, and Kolmogorov–Smirnov tests. We conducted a logarithmic transformation of skewed variables and confirmed normality before further analysis.
First, we examined the 15 through 24 yr cumulative independent and separate determinants or predictor of the 7-yr cfPWV and cIMT progression without adjusting for covariates. We then examined the separate prospective associations of the 9-yr progression (15 through 24 yr) of fasting insulin, glucose, and HOMA-IR with the longitudinal progression of each of cfPWV and cIMT measured from ages 17 through 24 yr using linear mixed-effect models for repeated measures with restricted maximum likelihood estimation. The estimates quantify the effect of the longitudinal progression of the predictors on the longitudinal progression of the outcome variables. We decided a priori to select the model with the least Bayesian information criterion. The optimal model was one with gender as a factor and a random intercept modeled on the subject level. We selected a scaled identity covariance type and determined the effect of the change in predictors on change in outcome variables. The mixed-effects models assume that the data are missing at random and is robust for accounting for missing data at follow-up. All analyses were adjusted for significant and independent determinants of either cfPWV or cIMT progression, from prospective determinant models, depending on the outcomes. We presented sex-based and BMI category-based results. We subsequently split the cumulative exposure to the predictor as 15 through 17 yr and 17 through 24 yr and each split was longitudinally associated with cfPWV and cIMT progression from 17 through 24 yr using the above statistical protocol for repeated linear mixed-effect models.
We performed collinearity diagnoses and accepted results with a variance inflation factor <5, considered differences and associations with a two-sided P value <0.05 as statistically significant, and made conclusions based on effect estimates and their confidence intervals (CI). A Sidak-corrected P value accounted for multiple comparison test. Analyses involving 20% of a sample of 10,000 ALSPAC children at 0.8 statistical power, 0.05 α, and two-sided P value would show a minimum detectable effect size of 0.062 standard deviations if they had relevant exposure for a normally distributed quantitative variable (24). All statistical analyses were performed using SPSS statistics software, v. 27.0 (IBM Corp, Armonk, NY).
RESULTS
Study Population and Characteristics
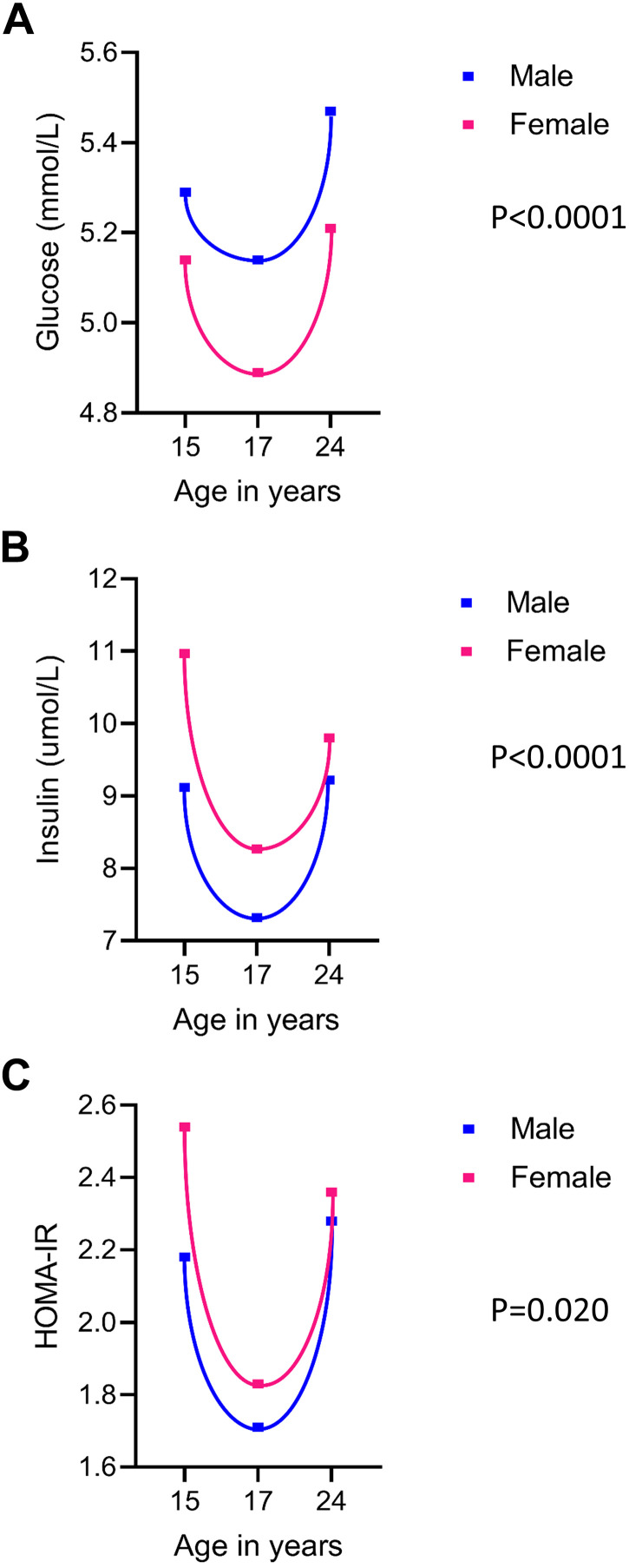
Of 14,901 children who were alive at 1 yr of age, 5,515 adolescents participated in the 15-yr follow-up, 5,217 adolescents participated in the 17-yr follow-up and 4,026 young adults participated in the 24-yr follow-up clinic visits (Supplemental Fig. S1). Only 1,779 participants that had complete fasting insulin and glucose measures at 15 and 17 yr and cfPWV measures at 17 yr were studied. From 15 through 24 yr, males had higher glucose concentrations than females however, females had higher insulin and HOMA-IR (Table 1, Fig. 1). We observed a steep decline in glucose, insulin, and HOMA-IR from ages 15 through 17 yr, which steadily increased from age 17 through 24 yr (Table 1, Fig. 1). Other characteristics are described in Table 1.
Table 1.
Descriptive characteristics of cohort participants
| 15 Yr |
17 Yr |
24 Yr |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variables | Male |
Female |
Male |
Female |
Male |
Female |
|||||||||
| N | Means (SD) | N | Means (SD) | P Value | N | Means (SD) | N | Means (SD) | P Value | N | Means (SD) | N | Means (SD) | P Value | |
| Anthropometry | |||||||||||||||
| Age, yr | 892 | 15.41 (0.27) | 887 | 15.44 (0.30) | 0.079 | 892 | 17.69 (0.29) | 887 | 17.69 (0.32) | 0.951 | 543 | 24.59 (0.74) | 686 | 24.47 (0.70) | 0.004 |
| Height, m | 885 | 1.75 (0.08) | 877 | 1.65 (0.06) | <0.0001 | 879 | 1.79 (0.07) | 874 | 1.66 (0.06) | <0.0001 | 546 | 1.80 (0.07) | 678 | 1.67 (0.06) | <0.0001 |
| *Weight, kg | 884 | 62.3 (12.8) | 874 | 57.9 (11.9) | <0.0001 | 881 | 70 (14.15) | 874 | 60.5 (13.63) | <0.0001 | 545 | 78.4 (17.05) | 677 | 65.2 (17.5) | <0.0001 |
| Attained puberty, n,% | 851 | 829 (97.4) | 836 | >831 (>99.4) | <0.0001 | 851 | >845 (>99.4) | 836 | >831 (>99.4) | NA | |||||
| Race—White, n,% | 820 | 785 (95.7) | 811 | 783 (96.5) | 0.441 | NA | NA | ||||||||
| Body composition | |||||||||||||||
| *Total fat mass, kg | 860 | 8.31 (6.92) | 851 | 17.32 (8.70) | <0.0001 | 874 | 10.28 (9.51) | 862 | 19.21 (9.96) | <0.0001 | 537 | 18.25 (10.59) | 660 | 21.74 (11.67) | <0.0001 |
| *Lean mass, kg | 860 | 50.3 (8.48) | 851 | 36.87 (4.85) | <0.0001 | 874 | 55.29 (8.17) | 862 | 38.01 (5.19) | <0.0001 | 537 | 56.64 (9.88) | 660 | 41.16 (6.77) | <0.0001 |
| *Body mass index, kg/m2 | 884 | 20.23 (3.29) | 874 | 21.13 (3.86) | <0.0001 | 879 | 21.57 (3.87) | 874 | 22.10 (4.50) | 0.004 | 545 | 24.14 (4.74) | 677 | 23.53 (5.80) | 0.233 |
| BMI category (overweight and obese), n,% | 884 | 70 (7.9) | 874 | 115 (13.2) | <0.0001 | 879 | 151 (17.2) | 874 | 192 (22.0) | 0.007 | 545 | 219 (40.2) | 677 | 247 (36.5) | 0.103 |
| Metabolic profile | |||||||||||||||
| Total cholesterol, mmol/L | 892 | 3.56 (0.59) | 887 | 3.90 (0.65) | <0.0001 | 892 | 3.56 (0.63) | 887 | 3.94 (0.69) | <0.0001 | 524 | 4.35 (0.85) | 611 | 4.47 (0.81) | 0.015 |
| High-density lipoprotein, mmol/L | 892 | 1.22 (0.27) | 887 | 1.35 (0.29) | <0.0001 | 892 | 1.19 (0.26) | 887 | 1.35 (0.32) | <0.0001 | 524 | 1.41 (0.36) | 611 | 1.66 (0.42) | <0.0001 |
| Low-density lipoprotein, mmol/L | 892 | 1.97 (0.52) | 887 | 2.16 (0.57) | <0.0001 | 892 | 1.99 (0.56) | 887 | 2.21 (0.64) | <0.0001 | 524 | 2.47 (0.80) | 611 | 2.40 (0.73) | 0.082 |
| *Triglyceride, mmol/L | 892 | 0.72 (0.37) | 887 | 0.77 (0.39) | <0.0001 | 892 | 0.74 (0.36) | 887 | 0.75 (0.36) | 0.182 | 524 | 0.87 (0.53) | 611 | 0.80 (0.43) | <0.0001 |
| Glucose, mmol/L | 892 | 5.29 (0.39) | 887 | 5.14 (0.38) | <0.0001 | 892 | 5.14 (0.42) | 887 | 4.89 (0.35) | <0.0001 | 524 | 5.47 (0.62) | 611 | 5.21 (0.51) | <0.0001 |
| *Insulin, mU/L | 892 | 8.09 (4.71) | 887 | 9.75 (5.84) | <0.0001 | 892 | 5.93 (3.92) | 887 | 7.29 (4.29) | <0.0001 | 524 | 7.02 (4.97) | 611 | 7.88 (5.41) | <0.0001 |
| *HOMA-IR | 892 | 1.92 (1.16) | 887 | 2.21 (1.41) | <0.0001 | 892 | 1.35 (0.96) | 887 | 1.60 (0.99) | <0.0001 | 524 | 1.68 (1.25) | 611 | 1.82 (1.34) | 0.043 |
| *High-sensitivity C-reactive protein, mg/L | 892 | 0.36 (0.59) | 887 | 0.37 (0.61) | 0.400 | 892 | 0.47 (0.72) | 887 | 0.66 (1.27) | <0.0001 | 466 | 0.63 (1.05) | 575 | 0.96 (1.94) | <0.0001 |
| Vascular measures | |||||||||||||||
| Pulse rate, beats/min | 866 | 71 (12) | 854 | 78 (12) | <0.0001 | 892 | 63 (9) | 886 | 67 (10) | <0.0001 | 548 | 64 (10) | 685 | 68 (10) | <0.0001 |
| Systolic blood pressure, mmHg | 867 | 126 (10) | 857 | 120 (10) | <0.0001 | 892 | 120 (9) | 886 | 110 (8) | <0.0001 | 548 | 123 (10) | 685 | 112 (9) | <0.0001 |
| Diastolic blood pressure, mmHg | 867 | 66 (9) | 857 | 66 (8) | 0.002 | 892 | 63 (9) | 886 | 64 (6) | <0.0001 | 548 | 67 (8) | 685 | 66 (8) | 0.038 |
| Hypertension, n,% | 867 | 8 (0.9) | 857 | 1 (0.1) | 0.020 | 892 | 3 (0.3) | 886 | 1 (0.1) | 0.315 | 548 | 2 (0.4) | 685 | 2 (0.3) | <0.600 |
| *Carotid-femoral PWV, m/s | NA | 892 | 5.99 (0.84) | 887 | 5.50 (0.71) | <0.0001 | 369 | 6.50 (1.23) | 502 | 5.87 (1.03) | <0.0001 | ||||
| *Carotid intima-media thickness, mm | NA | 888 | 0.48 (0.06) | 881 | 0.47 (0.06) | <0.0001 | 303 | 0.46 (0.06) | 430 | 0.45 (0.06) | 0.003 | ||||
| Lifestyle factors | |||||||||||||||
| Smoked cigarettes in the past 30 days, n,% | 867 | 101 (11.6) | 876 | 160 (18.3) | <0.0001 | 788 | 202 (25.6) | 785 | 209 (26.6) | 0.688 | 537 | 150 (27.9) | 681 | 174 (25.6) | 0.361 |
| Sedentary time, min/day | 427 | 462 (88) | 469 | 481 (81) | 0.001 | NA | 118 | 539 (80) | 216 | 523 (85) | 0.082 | ||||
| LPA, min/day | 427 | 294 (67) | 469 | 276 (61) | <0.0001 | NA | 118 | 143 (55) | 216 | 150 (53) | 0.305 | ||||
| MVPA, min/day | 427 | 57 (30) | 469 | 42 (23) | <0.0001 | NA | 118 | 54 (33) | 216 | 50 (28) | 0.076 | ||||
| Family history of H-D-C-V, n,% | NA | 891 | 248 (27.8) | 887 | 266 (30) | 0.321 | NA | ||||||||
The values are means (SD) and *median (interquartile range) except for puberty status, race, and lifestyle factors in percentage. Differences between sexes were tested using independent t test for normally distributed continuous variables, Mann–Whitney U test for skewed continuous variables, and chi-square test for dichotomous variables. A two-sided P value <0.05 is considered statistically significant. Participants at age 17 and 24 yr were classified as hypertensive if systolic and diastolic blood pressure is ≥140/90 mmHg, while at age 15 yr if systolic and diastolic blood pressure is ≥95th percentile according to the ESC/ESH hypertension guideline. P value for sex differences. BMI, body mass index; ESC, European Society of Cardiology; ESH, European Society of Hypertension; H-D-C-V, hypertension/diabetes/high cholesterol/vascular disease; HOMA-IR, homeostatic model assessment of insulin resistance; LPA, light physical activity; MVPA, moderate-to-vigorous physical activity; NA, not available/applicable; PWV, pulse wave velocity.
Figure 1.
Mean concentrations of metabolic indices during ages 15, 17, and 24 yr according to sex. Fasting glucose (A), fasting insulin (B), and homeostatic model assessment for insulin resistance (HOMA-IR; C). Sex difference was examined with independent t test for normally distributed variable (glucose) whereas Mann–Whitney U test was conducted for skewed variables (fasting insulin and HOMA-IR).
Determinants of Arterial Stiffness and cIMT Progression from Ages 17 to 24 Yr
The univariate cumulative determinants from ages 15 through 24 yr of increasing cfPWV progression were male sex, older age, increased high-sensitivity C-reactive protein, increased lean mass, increased sedentary time, and increased moderate-to-vigorous physical activity (Table 2). However, the independent cumulative determinants from ages 15 through 24 yr of decreasing cfPWV progression were increased high-density lipoprotein cholesterol, increased low-density lipoprotein cholesterol, increased insulin, increased HOMA-IR, increased total fat mass, increased heart rate, and increased light physical activity (Table 2).
Table 2.
Age 15 yr through 24 yr independent determinant of arterial stiffness and carotid intima-media thickness progression from adolescence (17 yr) through young adulthood (24 yr)
|
N = 1,779 |
Carotid-Femoral Pulse Wave Velocity |
Carotid Intima-Media Thickness |
||
|---|---|---|---|---|
| Univariate predictor | Effect estimate (95% CI) | P value | Effect estimate (95% CI) | P value |
| Sex (male) | 0.085 (0.074 to 0.096) | <0.0001 | 0.027 (0.019 to 0.035) | <0.0001 |
| Age | 0.031 (0.027 to 0.035) | <0.0001 | −0.016 (−0.019 to −0.013) | <0.0001 |
| High-density lipoprotein cholesterol | −0.045 (−0.064 to −0.026) | <0.0001 | −0.004 (−0.017 to 0.009) | 0.531 |
| Low-density lipoprotein cholesterol | −0.011 (−0.020 to −0.001) | 0.032 | 0.001 (−0.006 to 0.008) | 0.736 |
| *Triglyceride | −0.014 (−0.029 to 0.002) | 0.065 | 0.003 (−0.008 to 0.013) | 0.622 |
| *High-sensitivity C-reactive protein | 0.009 (0.004 to 0.014) | 0.001 | −0.004 (−0.007 to −0.0003) | 0.033 |
| *Insulin | −0.039 (−0.049 to −0.028) | <0.0001 | 0.010 (0.003 to 0.017) | 0.008 |
| Glucose | −0.001 (−0.014 to 0.013) | 0.945 | 0.029 (0.020 to 0.039) | <0.0001 |
| *HOMA-IR | −0.035 (−0.045 to −0.025) | <0.0001 | 0.012 (0.005 to 0.018) | 0.001 |
| Total fat mass | −0.016 (−0.026 to −0.006) | 0.001 | −0.018 (−0.025 to −0.011) | <0.0001 |
| Lean mass | 0.270 (0.242 to 0.298) | <0.0001 | 0.056 (0.036 to 0.077) | <0.0001 |
| Systolic blood pressure | 0.001 (<0.0001 to 0.001) | 0.068 | 0.002 (0.001 to 0.002) | <0.0001 |
| Diastolic blood pressure | 0.001 (−0.0002 to 0.001) | 0.233 | 0.001 (0.0003 to 0.001) | 0.002 |
| Heart rate | −0.002 (−0.003 to −0.002) | <0.0001 | <0.0001 (−0.0002 to 0.001) | 0.564 |
| Sedentary time | 0.0001 (0.0001 to 0.0002) | 0.011 | −0.0001 (−0.0002 to −0.0001 | <0.0001 |
| Light physical activity | −0.0003 (−0.0003 to −0.0002) | <0.0001 | 0.0002 (0.0001 to 0.0003) | <0.0001 |
| Moderate-to-vigorous physical activity | 0.001 (0.0002 to 0.001) | 0.001 | 0.0001 (−0.0001 to 0.0003) | 0.278 |
| Socioeconomic status | −0.007 (−0.013 to 0.0001) | 0.051 | −0.001 (−0.005 to 0.004) | 0.810 |
| Family history of cardiometabolic disease | −0.006 (−0.019 to 0.007) | 0.383 | 0.004 (−0.005 to 0.013) | 0.385 |
| Smoking status | −0.002 (−0.017 to 0.013) | 0.770 | 0.011 (0.001 to 0.022) | 0.028 |
| Puberty | −0.005 (−0.057 to 0.047) | 0.850 | −0.026 (−0.062 to 0.011) | 0.168 |
Skewed variables were logarithmically transformed. β is unstandardized regression coefficient from linear mixed-effect model analyses. Associations with P values <0.05 are considered statistically significant. CI, confidence interval; HOMA-IR, homeostatic model assessment of insulin resistance.
The independent cumulative determinants from ages 15 through 24 yr of increased cIMT progression were male sex, increased insulin, increased glucose, increased HOMA-IR, increased lean mass, increased systolic blood pressure, increased diastolic blood pressure, increased light physical activity, and increased smoking (Table 2). However, from ages 15 through 24 yr, independent cumulative determinants of decreased cIMT progression were older age, increased high-sensitivity C-reactive protein, increased total fat mass, and increased sedentary time (Table 2).
Cumulative Effect of High Exposure to Fasting Glucose, Insulin, and Insulin Resistance from Ages 15 to 24 Yr on cfPWV and cIMT Progression from Ages 17 to 24 Yr
When compared with the moderate or reference category, cumulative high insulin and HOMA-IR from age 15 through 24 yr were separately associated with optimal or healthy cfPWV progression after adjusting for statistically significant covariates (Table 3). There were no statistically significant associations between cumulative glucose concentration and cfPWV progression. Cumulative insulin, glucose and HOMA-IR were unassociated with cIMT progression (Table 3). According to sex and BMI categories, cumulative insulin, glucose, and HOMA-IR had no statistically significant associations with either cfPWV or cIMT progression (Tables 3 and 4).
Table 3.
Cumulative effect of insulin and insulin resistance categories from age 15 to 24 yr on carotid-femoral pulse wave velocity and carotid intima-media thickness progression from ages 17 through 24 yr
|
N = 1,799 |
Carotid-Femoral Pulse Wave Velocity |
Carotid Intima-Media Thickness |
||
|---|---|---|---|---|
| All participants | Effect estimate (95% CI) | P value | Effect estimate (95% CI) | P value |
| High glucose (≥75 percentile) | Reference | Reference | ||
| 0.008 (−0.008 to 0.025) | 0.302 | 0.009 (−0.003 to 0.021) | 0.130 | |
| High insulin (≥75 percentile) | Reference | Reference | ||
| −0.019 (−0.037 to −0.002) | 0.033 | 0.002 (−0.030 to 0.013) | 0.704 | |
| High HOMA-IR (≥75 percentile) | Reference | Reference | ||
| −0.021 (−0.039 to −0.004) | 0.019 | 0.003 (−0.009 to 0.016) | 0.608 | |
| Male (n = 892) | ||||
| High glucose (≥75 percentile) | Reference | Reference | ||
| 0.017 (−0.008 to 0.042) | 0.183 | 0.004 (−0.014 to 0.022) | 0.656 | |
| High insulin (≥75 percentile) | Reference | Reference | ||
| −0.017 (−0.045 to 0.011) | 0.221 | −0.001 (−0.020 to 0.019) | 0.910 | |
| High HOMA-IR (≥75 percentile) | Reference | Reference | ||
| −0.022 (−0.050 to 0.006) | 0.119 | 0.003 (−0.016 to 0.023) | 0.738 | |
| Female (n = 887) | ||||
| High glucose (≥75 percentile) | Reference | Reference | ||
| 0.002 (−0.019 to 0.023) | 0.849 | 0.011 (−0.005 to 0.026) | 0.191 | |
| High insulin (≥75 percentile) | Reference | Reference | ||
| −0.020 (−0.044 to 0.003) | 0.085 | 0.007 (−0.011 to 0.024) | 0.461 | |
| High HOMA-IR (≥75 percentile) | Reference | Reference | ||
| −0.020 (−0.043 to 0.003) | 0.085 | 0.004 (−0.013 to 0.022) | 0.630 | |
Effect estimates and confidence interval (CI) from linear mixed model repeated-measure analyses. Associations with P values <0.05 are considered statistically significant. The model that included all participants was adjusted for all statistically significant univariate predictors of arterial stiffness progression, namely, sex, and age, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, high-sensitivity C-reactive protein, total fat mass, lean mass, heart rate, sedentary time, light physical activity, and moderate-to-vigorous physical activity. The association of fasting glucose with arterial stiffness progression was additionally adjusted for insulin. Carotid intima-media thickness progression model was adjusted for all statistically significant univariate predictors, namely, sex, age, high-sensitivity C-reactive protein, insulin or glucose, total fat mass, lean mass, systolic blood pressure, diastolic blood pressure, sedentary time, light physical activity, and smoking status. HOMA-IR model was not adjusted for insulin or glucose. All predictors and covariates except sex were repeatedly measured at 15-, 17-, and 24-yr clinic visits. Sex-based analyses were not adjusted for sex. The outcomes, carotid-femoral pulse wave velocity and carotid intima-media thickness, were measured at 17- and 24-yr clinic visits. Glucose, insulin, and insulin resistance binary categories were grouped as ≥75 percentile as high and <75 percentile as moderate, normal, healthy, or not high. The moderate, normal or healthy group was the reference category. HOMA-IR, homeostatic model assessment of insulin resistance.
Table 4.
Body mass index stratified groups of cumulative effect of insulin and insulin resistance categories from age 15 to 24 yr on carotid-femoral pulse wave velocity and carotid intima-media thickness progression from ages 17 through 24 yr
| BMI-Groups at 17 yr of age |
Carotid-Femoral Pulse Wave Velocity |
Carotid Intima-Media Thickness |
||
|---|---|---|---|---|
| Normal Weight (n = 1,430) | Effect Estimate (95% CI) | P Value | Effect Estimate (95% CI) | P Value |
| High glucose (≥75 percentile) | Reference | Reference | ||
| 0.054 (0.027 to 0.081) | 0.465 | 0.011 (−0.002 to 0.025) | 0.089 | |
| High insulin (≥75 percentile) | Reference | Reference | ||
| −0.018 (−0.038 to 0.002) | 0.079 | 0.002 (−0.013 to 0.017) | 0.788 | |
| High HOMA-IR (≥75 percentile) | Reference | Reference | ||
| −0.019 (−0.039 to 0.002) | 0.072 | 0.003 (−0.011 to 0.018) | 0.666 | |
| Overweight and obese (n = 349) | ||||
| High glucose (≥75 percentile) | Reference | Reference | ||
| 0.008 (−0.028 to 0.044) | 0.674 | −0.002 (−0.029 to 0.025) | 0.879 | |
| High insulin (≥75 percentile) | Reference | Reference | ||
| −0.013 (−0.048 to 0.022) | 0.469 | −0.003 (−0.029 to 0.023) | 0.823 | |
| High HOMA-IR (≥75 percentile) | Reference | Reference | ||
| −0.020 (−0.055 to 0.014) | 0.249 | 0.0002 (−0.025 to 0.025) | 0.990 | |
| BMI—groups at 24 yr of age | ||||
| Normal weight (n = 1,103) | ||||
| High glucose (≥75 percentile) | Reference | Reference | ||
| −0.003 (−0.026 to 0.019) | 0.764 | 0.016 (−0.001 to 0.032) | 0.061 | |
| High insulin (≥75 percentile) | Reference | Reference | ||
| −0.005 (−0.030 to 0.021) | 0.726 | 0.013 (−0.005 to 0.032) | 0.160 | |
| High HOMA-IR (≥75 percentile) | Reference | Reference | ||
| −0.012 (−0.037 to 0.013) | 0.360 | 0.013 (−0.005 to 0.032) | 0.154 | |
| Overweight and obese (n = 676) | ||||
| High glucose (≥75 percentile) | Reference | Reference | ||
| 0.025 (−0.007 to 0.057) | 0.130 | 0.013 (−0.010 to 0.035) | 0.269 | |
| High insulin (≥75 percentile) | Reference | Reference | ||
| −0.027 (−0.058 to 0.005) | 0.099 | −0.010 (−0.031 to 0.115) | 0.362 | |
| High HOMA-IR (≥75 percentile) | Reference | Reference | ||
| −0.024 (−0.055 to 0.007) | 0.129 | −0.008 (−0.028 to 0.014) | 0.482 | |
Effect estimates and confidence interval (CI) from linear mixed model repeated-measure analyses. Associations with P values <0.05 are considered statistically significant. The model which included all participants was adjusted for all statistically significant univariate predictors of arterial stiffness progression, namely, sex, age, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, high-sensitivity C-reactive protein, lean mass, heart rate, sedentary time, light physical activity, and moderate-to-vigorous physical activity. The association of fasting glucose with arterial stiffness progression was additionally adjusted for insulin. Carotid intima-media thickness progression model was adjusted for all statistically significant univariate predictors, namely, sex, age, high-sensitivity C-reactive protein, insulin or glucose, lean mass, systolic blood pressure, diastolic blood pressure, sedentary time, light physical activity, and smoking status. HOMA-IR model was not adjusted for insulin or glucose. All predictors and covariates except sex were repeatedly measured at 15-, 17-, and 24-yr clinic visits. The outcomes, carotid-femoral pulse wave velocity and carotid intima-media thickness, were measured at 17- and 24-yr clinic visit. Glucose, insulin, and insulin resistance binary categories were grouped as ≥75 percentile as high and <75 percentile as moderate, normal, healthy. The moderate, normal, or healthy group was the reference category. Body mass index category was classified as ≥ 25 kg/m2 as overweight and obese and those with <25 kg/m2 as normal weight. BMI, body mass index; CI; confidence interval; HOMA-IR, homeostatic model assessment of insulin resistance.
Cumulative Effect of High Exposure to Fasting Glucose, Insulin, and Insulin Resistance from Ages 15 to 17 Yr on cfPWV and cIMT Progression from Ages 17 to 24 Yr
When compared with the moderate or reference category, cumulative high insulin and HOMA-IR from age 15 through 17 yr were associated with healthy or optimal cfPWV progression, especially in females (Table 5). There were no statistically significant associations between cumulative glucose concentration and cfPWV progression in males and females. Cumulative insulin, glucose, and HOMA-IR were unassociated with cIMT progression among all participants, either in males or females (Table 5).
Table 5.
Cumulative effect of insulin and insulin resistance categories from age 15 to 17 yr or 17 to 24 yr on carotid-femoral pulse wave velocity and carotid intima-media thickness progression from ages 17 through 24 yr
|
N = 1,799 |
Carotid-Femoral Pulse Wave Velocity |
Carotid Intima-Media Thickness |
||
|---|---|---|---|---|
| 15 to 17 yr | Effect Estimate (95% CI) | P Value | Effect Estimate (95% CI) | P Value |
| High glucose (≥75 percentile) | Reference | Reference | ||
| 0.005 (−0.010 to 0.021) | 0.494 | 0.004 (−0.008 to 0.015) | 0.530 | |
| High insulin (≥75 percentile) | Reference | Reference | ||
| −0.025 (−0.042 to −0.009) | 0.003 | −0.003 (−0.015 to 0.010) | 0.668 | |
| High HOMA-IR (≥75 percentile) | Reference | Reference | ||
| −0.023 (−0.039 to −0.007) | 0.006 | −0.0003 (−0.012 to 0.012) | 0.960 | |
| Male (n = 892) | ||||
| High glucose (≥75 percentile) | Reference | Reference | ||
| 0.010 (−0.013 to 0.033) | 0.400 | 0.006 (−0.011 to 0.023) | 0.503 | |
| High insulin (≥75 percentile) | Reference | Reference | ||
| −0.020 (−0.046 to 0.005) | 0.121 | −0.010 (−0.028 to 0.010) | 0.347 | |
| High HOMA-IR (≥75 percentile) | Reference | Reference | ||
| −0.022 (−0.047 to 0.004) | 0.094 | −0.004 (−0.023 to 0.014) | 0.639 | |
| Female (n = 887) | ||||
| High glucose (≥75 percentile) | Reference | Reference | ||
| 0.001 (−0.020 to 0.022) | 0.905 | −0.001 (−0.017 to 0.014) | 0.916 | |
| High insulin (≥75 percentile) | Reference | Reference | ||
| −0.031 (−0.053 to −0.009) | 0.006 | 0.004 (−0.012 to 0,020) | 0.615 | |
| High HOMA-IR (≥75 percentile) | Reference | Reference | ||
| −0.026 (−0.048 to −0.004) | 0.021 | 0.004 (−0.012 to 0.020) | 0.598 | |
| 17 to 24 yr—all participants (N = 1,799) | ||||
| High glucose (≥75 percentile) | Reference | Reference | ||
| −0.006 (−0.033 to 0.020) | 0.637 | −0.006 (−0.024 to 0.013) | 0.559 | |
| High insulin (≥75 percentile) | Reference | Reference | ||
| −0.010 (−0.037 to 0.018) | 0.482 | −0.026 (−0.045 to −0.006) | 0.009 | |
| High HOMA-IR (≥75 percentile) | Reference | Reference | ||
| −0.004 (−0.032 to 0.024) | 0.776 | −0.021 (−0.040 to −0.002) | 0.031 | |
| Male (n = 892) | ||||
| High glucose (≥75 percentile) | Reference | Reference | ||
| −0.003 (−0.048 to 0.042) | 0.890 | −0.006 (−0.037 to 0.026) | 0.721 | |
| High insulin (≥75 percentile) | Reference | Reference | ||
| −0.008 (−0.055 to 0.040) | 0.752 | −0.042 (−0.074 to −0.011) | 0.008 | |
| High HOMA-IR (≥75 percentile) | Reference | Reference | ||
| 0.002 (−0.046 to 0.050) | 0.936 | −0.041 (−0.072 to −0.010) | 0.010 | |
| Female (n = 887) | ||||
| High glucose (≥75 percentile) | Reference | Reference | ||
| −0.004 (−0.037 to 0.030) | 0.826 | −0.017 (−0.040 to 0.006) | 0.146 | |
| High insulin (≥75 percentile) | Reference | Reference | ||
| −0.013 (−0.047 to 0.022) | 0.479 | −0.009 (−0.032 to 0.016) | 0.493 | |
| High HOMA-IR (≥75 percentile) | Reference | Reference | ||
| −0.008 (−0.043 to 0.026) | 0.635 | −0.007 (−0.030 to 0.017) | 0.581 | |
Effect estimates and confidence interval (CI) from linear mixed model repeated-measure analyses. Associations with P values <0.05 are considered statistically significant. The model which included all participants was adjusted for all statistically significant univariate predictors of arterial stiffness progression, namely, sex, and age, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, high-sensitivity C-reactive protein, total fat mass, lean mass, heart rate, sedentary time, light physical activity, and moderate-to-vigorous physical activity. The association of fasting glucose with arterial stiffness progression was additionally adjusted for insulin. Carotid intima-media thickness progression model was adjusted for all statistically significant univariate predictors, namely, sex, age, high-sensitivity C-reactive protein, insulin or glucose, total fat mass, lean mass, systolic blood pressure, diastolic blood pressure, sedentary time, light physical activity, and smoking status. HOMA-IR model was not adjusted for insulin or glucose. All predictors and covariates except sex were repeatedly measured at 15-, 17-, and 24-yr clinic visits. The outcomes, carotid-femoral pulse wave velocity and carotid intima-media thickness, were measured at 17- and 24-yr clinic visits. Insulin and insulin resistance binary categories were grouped as ≥75 percentile as high and <75 percentile as moderate. normal healthy, or not high. The moderate, normal, or healthy group was the reference category. HOMA-IR, homeostatic model assessment of insulin resistance.
Cumulative Effect of High Exposure to Fasting Glucose, Insulin, and Insulin Resistance from Ages 17 to 24 Yr on cfPWV and cIMT Progression from Ages 17 to 24 Yr
Cumulative glucose, insulin, and HOMA-IR from age 17 through 24 yr had no statistically significant associations with cfPWV progression, in males and females (Table 5). However, cumulative insulin and HOMA-IR from ages 17 to 24 yr were separately associated with healthy or optimal cIMT progression in males but not in females (Table 5). Cumulative glucose was not associated with cIMT progression.
DISCUSSION
In a population-based healthy cohort, we found that fasting glucose, insulin, HOMA-IR decreased and later increased in a J- or U-shaped form during growth and maturation from ages 15 to 24 yr. Moreover, the cumulative determinants of cfPWV and cIMT progression were divergent. For the first time, we showed in a longitudinal study that accumulated exposures to high insulin and HOMA-IR from ages 15 to 24 yr independently predicted slower cfPWV progression during ages 17–24 yr. The seemingly protective association of cumulative insulin and HOMA-IR on cfPWV progression appears to be driven by the physiologic decline in glucose, insulin, and HOMA-IR between ages 15 through 17 yr, especially in females. However, cumulative high glucose, insulin, and HOMA-IR from ages 15 to 24 yr were not associated with cIMT progression, after controlling for covariates.
A previous review of mainly cross-sectional reports among healthy and type 2 diabetic adults reported that insulin resistance contributes to increased arterial stiffness independent of blood pressure (6). A longitudinal study among Japanese adult men with a mean age of 42 yr and followed up for 3 yr, concluded that fasting glucose was strongly associated with arterial stiffness progression (7). Another longitudinal study from the Framingham cohort whose participants’ mean age was 50 yr and followed up for 6 yr, also reported that change in glucose was associated with arterial stiffness progression (8). However, there is a paucity of prospective studies among healthy adolescents which would help clarify how physiologic arterial changes associate with metabolic changes. Recently, a longitudinal study among 304 adolescents with an 8-yr mean duration of diagnosed type 2 diabetes mellitus concluded that higher cumulative glycemic exposure was associated with the 5-yr worsening progression in arterial stiffness (9). The study also reported that the annual change in cfPWV was 0.15 m/s; however, the study is limited in that they did not account for important risk factors such as levels of physical activity (9).
Among our study cohort of 1,779 healthy adolescents whose vascular profiles were followed up for 7 yr, the annual change in cfPWV was 0.06 m/s. This is approximately one-third of the rate of cfPWV increase in adolescents with type 2 diabetes (9). It is, therefore, unsurprising that cumulative glycemic exposure was associated with cfPWV progression in that population (9) but not among our study participants. Intriguingly, we observed a protective effect of cumulative insulin and HOMA-IR on cfPWV progression mainly explained by the steep decline in the cumulative exposure between ages 15 to 17 yr, especially among females. The protective effect of insulin on arterial stiffness progression seen among females may be due to the complementary and vasodilatory effect of sex steroid hormones on elastic arteries, postpuberty (25). The adverse effect of insulin resistance on arterial stiffness among obese adolescence remains unclear due to the scarcity of longitudinal evidence (26, 27). Cross-sectional studies have reported that HOMA-IR was not associated with cfPWV in healthy adolescents (10, 11). These studies (10, 11) could not account for the concurrent vascular and metabolic changes due to growth and maturation; therefore, inferences drawn from cross-sectional reports are limited to hypothesis generation.
When we stratified by BMI category of normal weight, overweight and/or obese at 17 or 24 yr, we found no association between metabolic indices and arterial function and structure. This is congruent with our findings that the cumulative increase in total fat mass was negatively associated with cfPWV progression (28). In comparison with adolescents with normal weight, obesity does not seem to contribute to arterial stiffness progression both in our study and a previous prospective study (5, 27, 28). It is however surprising that cumulative moderate-to-vigorous physical activity was independently associated with increasing or worsening cfPWV progression. A brief explanation could be that participants who were regularly involved in moderate-to-vigorous physical activity may have developed vascular adaptation (stiffer arteries) to exercise. This phenomenon was previously reported among long career athletes (29). On the contrary, light physical activity appears to be protective of cfPWV progression although the relationship was attenuated after multivariate adjustments, leaving only sex, age, and lean mass as consistent cumulative determinants of cfPWV progression as recently reported (28). Low-grade inflammation may be a determinant of arterial stiffness progression and a recent temporal causal study suggests that inflammation may precede premature vascular aging and damage (30). Future study may examine whether inflammation mediates the relationships between metabolic changes in adolescence and arterial stiffness progression.
Evidence on the longitudinal association of cumulative metabolic profile on repeated measures of cIMT among healthy adolescents is limited (27). Among youth with obesity, insulin resistance was associated with higher cIMT during a 2-yr follow-up (31). A recent study found no relationship between metabolic indices and cIMT progression in 141 healthy adolescents aged 17 yr and followed up for 5 yr (27). Similarly, among 1,779 healthy adolescents followed up for 9 yr, we observed that cumulative metabolic indices from ages 15 through 24 yr were not associated with cIMT progression, either based on sex or BMI weight categories. However, we observed that the steady increase in insulin and HOMA-IR from age 17 through 24 yr was negatively associated with cIMT progression, particularly among males. A previous longitudinal study concluded that the male sex is an independent and primary risk factor for accelerated cIMT increase (27). The disparity in our findings may be that our study has a larger sample size [13 times more healthy adolescents than the previous study (27)], a longer follow-up, a homogenous study population, higher statistical power, better statistical modeling approach that uses raw values rather than delta-change, and gold-standard characterization of important covariates such as body composition, and physical activity.
Arterial stiffness and cIMT are strong predictors of cardiovascular morbidities and mortality and efforts targeted at preventing or reducing their progression from early life has public health and clinical significance (1, 2, 4, 32). It is known that insulin has acute vasodilatory effects, which could lower arterial stiffening, although this benefit may be blunted in insulin-resistant states such as obesity (6, 33). In diabetic individuals, advanced glycation end-products form on the arterial wall, which leads to increased collagen molecules cross-link, thereby increasing the collagen-elastin ratio and ultimately stiffer arteries (34). Moreover, persistent hyperglycaemia and hyperinsulinaemia lead to overexpression of angiotensin type I receptors in vascular tissue, resulting in hypertrophy and fibrosis within the arterial wall (35). Considering that our adolescents were apparently healthy with less than 0.5% diagnosed with type 2 diabetes mellitus and 10% were obese, cumulative metabolic changes did not reflect worsening arterial stiffness or cIMT progression. Since moderate-to-vigorous physical activity may lead to increase lean mass and higher metabolism, a stepwise adjustment for these factors did not alter the association between cumulative insulin and cfPWV progression.
In our cohort, arterial stiffness progression from adolescence through young adulthood appears to be physiologically delayed by a decline in insulin resistance during mid-adolescence. In further analyses using continuous variables of insulin and HOMA-IR (data not shown) the direction of the associations of cumulative insulin and HOMA-IR at either age 15 through 17 yr or 17 through 24 yr with arterial stiffness progression were contrasting. Cumulative insulin and HOMA-IR from ages 15 to 17 yr were negatively associated with increasingly high cfPWV whereas the progression in insulin and HOMA-IR from ages 17 to 24 yr was positively associated with increasingly high cfPWV. Nonetheless, further research is warranted to explain the mechanism of the physiologic decline in insulin, glucose, and HOMA-IR from ages 15 to 17 yr and the steady rise from 17 through 24 yr. The J-shaped and U-shaped increase in glucose, insulin, and HOMA-IR during the transition from mid-adolescence through young adulthood was recently reported in a Finnish randomized controlled trial both in the intervention and controlled group (36). This steady metabolic decline was consistent with the decrease in heart rate and blood pressure, however, body composition (lean mass and fat mass) and lipid profile, high-sensitivity C-reactive protein, smoking status increased (37). Late adolescence appears to be a critical window of public health and clinical significance where efforts can be targeted at limiting the sharp increase in glucose, insulin, and HOMA-IR. Such intervention may sustain the protective effect of metabolic indices on arterial profile which was gained with the physiologic decline in metabolic indices between ages 15 to 17 yr (36). This is important since we recently showed that late adolescent arterial stiffness may be causally associated with insulin resistance and hyperinsulinemia in young adulthood (4). Moreover, cumulative dyslipidaemia has been associated with worsening cIMT progression in the same age group independent of cumulative fasting glucose and insulin (37).
Strengths and Limitations
We present the largest longitudinal study to date that examined the repeated measures of metabolic indices with gold-standard repeated measures of arterial outcomes using ALSPAC data. The availability of measurement from mid-adolescence (15 yr) through young adulthood (24 yr) provides an understanding of the physiological role of metabolism on arterial health during growth and maturation in a healthy population. Accounting for an objectively measured sedentary time and physical activity behavior rather than self-reported physical activity measure is one of the study’s strengths. We also serially measured fat mass and lean mass using dual-energy-X-ray absorptiometry at 15, 17, and 24 yr. Although almost all the participants have attained puberty at 15 yr, we could not control for the effect of changes in hormones on arterial health because hormonal variables were unavailable. Nonetheless, our findings revealed that attainment of objectively assessed puberty may not be a determinant of vascular progression in the non-adjusted model, in tandem with a previous report in the same cohort (38). We used HOMA-IR which is moderately correlated with the gold-standard measure of insulin resistance since the hyperinsulinemic-euglycemic clamp measure of insulin resistance was unavailable (22). Nonetheless, the single batch fasting sample analyses and narrow coefficient of variation reported in our cohort may increase the clinical usefulness of our findings (1, 16, 38). There were no regional differences in our study population as all were from the Avon area of England and were mostly White participants; therefore, we are unable to generalize our findings to other populations with different ethnicities. Future studies aimed at replicating these findings are warranted in other ethnic groups. Lastly, the observational nature of our study may not establish causality but recent temporal causal evidence and longitudinal studies in youth increases the strength of the present findings (4, 30, 37).
Conclusions
The cumulative insulin and HOMA-IR from mid-adolescence through young adulthood were negatively associated with arterial stiffness but not cIMT progression. The relationship between age and metabolic indices is U-shaped with a decrease in metabolic indices observed between age 15 and 17 yr, and a subsequent increase from 17 to 24 yr of age. The steady decline in insulin and HOMA-IR from ages 15 to 17 yr seems to physiologically limit arterial stiffness progression. However, the increase in insulin and HOMA-IR from ages 17 to 24 yr was not associated with arterial stiffness progression. The overall associations of insulin and HOMA-IR from ages 15 to 24 yr was negatively associated with arterial stiffness progression from ages 17 to 24 yr. Efforts targeted at retaining metabolic concentrations values observed during late adolescence (around age 17 yr) may promote arterial health and slow arterial stiffness progression.
DATA AVAILABILITY
The informed consent obtained from ALSPAC participants does not allow the data to be made freely available through any third-party maintained public repository. However, data used for this submission can be made available on request to the ALSPAC Executive. The ALSPAC data management plan describes in detail the policy regarding data sharing, which is through a system of managed open access. Full instructions for applying for data access can be found here: http://www.bristol.ac.uk/alspac/researchers/access/. The ALSPAC study website contains details of all the data that are available (http://www.bristol.ac.uk/alspac/researchers/our-data/).
SUPPLEMENTAL DATA
Supplemental Fig. S1 and Supplemental Table S1: https://doi.org/10.6084/m9.figshare.21997502.v1.
GRANTS
The UK Medical Research Council and Wellcome (Grant ref: 217065/Z/19/Z) and the University of Bristol provide core support for ALSPAC. The British Heart Foundation grant (CS/15/6/31468) funded blood pressure, carotid intima-media thickness, carotid-femoral pulse wave velocity, and Actigraph activity monitoring device measurement at 24 yr. The Medical Research Council grant (MR/M006727/1) supported smoking data collection. A comprehensive list of grant funding is available on the ALSPAC website (http://www.bristol.ac.uk/alspac/external/documents/grant-acknowledgements.pdf). This publication is the work of the authors and A.O.A. and T.-P.T. will serve as guarantors for the contents of this paper. Dr Agbaje's research group (UndeRstanding FITness and Cardiometabolic Health In Little Darlings: urFIT-child) was funded by the Jenny and Antti Wihuri Foundation (Grant no: 00180006); the North Savo regional and central Finnish Cultural Foundation (Grants no: 65191835 and 00200150); Orion Research Foundation sr, Aarne Koskelo Foundation, Antti and Tyyne Soininen Foundation, Paulo Foundation, Paavo Nurmi Foundation, Yrjö Jahnsson Foundation (Grant no: 20217390); and the Finnish Foundation for Cardiovascular Research (Grant no: 220021). Dr Zacharias was funded by National Heart, Lung, and Blood Institute (Grant no: R01 HL148217). The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. Drs. Agbaje and Tuomainen had full access to all the data in the study and take responsibility for the integrity of the data, accuracy of the data analysis and are guarantors for this work.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.O.A. conceived and designed research; A.O.A. performed experiments; A.O.A. analyzed data; A.O.A. and J.P.Z. interpreted results of experiments; A.O.A. prepared figures; A.O.A. drafted manuscript; A.O.A., J.P.Z., O.B., A.N.O., and T.-P.T. edited and revised manuscript; A.O.A., J.P.Z., O.B., A.N.O., and T.-P.T. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors are extremely grateful to all the families who took part in this study, the midwives for their help in recruiting them, and the whole ALSPAC team, which includes interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, receptionists and nurses. Graphical abstract was created with BioRender.com and published with permission.
REFERENCES
- 1. Agbaje AO, Barker AR, Tuomainen T-P. Effects of arterial stiffness and carotid intima-media thickness progression on the risk of overweight/obesity and elevated blood pressure/hypertension: a cross-lagged cohort study. Hypertension 79: 159–169, 2022. doi: 10.1161/HYPERTENSIONAHA.121.18449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chirinos JA, Segers P, Hughes T, Townsend R. Large-artery stiffness in health and disease: JACC state-of-the-art review. J Am Coll Cardiol 74: 1237–1263, 2019. doi: 10.1016/j.jacc.2019.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness. A systematic review and meta-analysis. J Am Coll Cardiol 55: 1318–1327, 2010. doi: 10.1016/j.jacc.2009.10.061. [DOI] [PubMed] [Google Scholar]
- 4. Agbaje AO, Barker AR, Mitchell GF, Tuomainen T-P. Effect of arterial stiffness and carotid intima-media thickness progression on the risk of dysglycemia, insulin resistance, and dyslipidemia: a temporal causal longitudinal study. Hypertension 79: 667–678, 2022. doi: 10.1161/HYPERTENSIONAHA.121.18754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Agbaje AO. Arterial stiffness precedes hypertension and metabolic risks in youth: a review. J Hypertens 40: 1887–1896, 2022. doi: 10.1097/HJH.0000000000003239. [DOI] [PubMed] [Google Scholar]
- 6. Stehouwer CDA, Henry RMA, Ferreira I. Arterial stiffness in diabetes and the metabolic syndrome: a pathway to cardiovascular disease. Diabetologia 51: 527–539, 2008. doi: 10.1007/s00125-007-0918-3. [DOI] [PubMed] [Google Scholar]
- 7. Tomiyama H, Hashimoto H, Hirayama Y, Yambe M, Yamada J, Koji Y, Shiina K, Yamamoto Y, Yamashina A. Synergistic acceleration of arterial stiffening in the presence of raised blood pressure and raised plasma glucose. Hypertension 47: 180–188, 2006. doi: 10.1161/01.HYP.0000198539.34501.1a. [DOI] [PubMed] [Google Scholar]
- 8. Zachariah JP, Rong J, Larson MG, Hamburg NM, Benjamin EJ, Vasan RS, Mitchell GF. Metabolic predictors of change in vascular function: prospective associations from a community-based cohort. Hypertension 71: 237–242, 2018. doi: 10.1161/HYPERTENSIONAHA.117.10054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shah AS, El Ghormli L, Gidding SS, Hughan KS, Levitt Katz LE, Koren D, Tryggestad JB, Bacha F, Braffett BH, Arslanian S, Urbina EM; TODAY Study Group. Longitudinal changes in vascular stiffness and heart rate variability among young adults with youth-onset type 2 diabetes: results from the follow-up observational treatment options for type 2 diabetes in adolescents and youth (TODAY) study. Acta Diabetol 59: 197–205, 2022. doi: 10.1007/s00592-021-01796-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zachariah JP, Wang Y, Newburger JW, Deferranti SD, Mitchell GF, Vasan RS. Biological pathways in adolescent aortic stiffness. J Am Heart Assoc 10: e018419, 2021. doi: 10.1161/JAHA.120.018419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Urbina EM, Gao Z, Khoury PR, Martin LJ, Dolan LM. Insulin resistance and arterial stiffness in healthy adolescents and young adults. Diabetologia 55: 625–631, 2012. doi: 10.1007/s00125-011-2412-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O'Neal L, McLeod L, Delacqua G, Delacqua F, Kirby J, Duda SN; REDCap Consortium. The REDCap consortium: building an international community of software platform partners. J Biomed Inform 95: 103208, 2019. doi: 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Boyd A, Golding J, Macleod J, Lawlor DA, Fraser A, Henderson J, Molloy L, Ness A, Ring S, Smith GD. Cohort profile: The ‘children of the 90s’-the index offspring of the avon longitudinal study of parents and children. Int J Epidemiol 42: 111–127, 2013. doi: 10.1093/ije/dys064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fraser A, Macdonald-Wallis C, Tilling K, Boyd A, Golding J, Davey Smith G, Henderson J, Macleod J, Molloy L, Ness A, Ring S, Nelson SM, Lawlor DA. Cohort profile: the avon longitudinal study of parents and children: ALSPAC mothers cohort. Int J Epidemiol 42: 97–110, 2013. doi: 10.1093/ije/dys066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Northstone K, Lewcock M, Groom A, Boyd A, Macleod J, Timpson N, Wells N. The Avon Longitudinal Study of Parents and Children (ALSPAC): an update on the enrolled sample of index children in 2019. Wellcome Open Res 4: 51, 2019. doi: 10.12688/wellcomeopenres.15132.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dangardt F, Charakida M, Georgiopoulos G, Chiesa ST, Rapala A, Wade KH, Hughes AD, Timpson NJ, Pateras K, Finer N, Sattar N, Davey Smith G, Lawlor DA, Deanfield JE. Association between fat mass through adolescence and arterial stiffness: a population-based study from The Avon Longitudinal Study of Parents and Children. Lancet Child Adolesc Health 3: 474–481, 2019. doi: 10.1016/S2352-4642(19)30105-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Frysz M, Howe LD, Tobias JH, Paternoster L. Using SITAR (Superimposition by translation and rotation) to estimate age at peak height velocity in Avon Longitudinal Study of Parents and Children [version 2; referees: 2 approved]. Wellcome Open Res 3: 90, 2018. doi: 10.12688/wellcomeopenres.14708.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, Clement DL, Coca A, de Simone G, Dominiczak A, Kahan T, Mahfoud F, Redon J, Ruilope L, Zanchetti A, Kerins M, Kjeldsen SE, Kreutz R, Laurent S, Lip GYH, McManus R, Narkiewicz K, Ruschitzka F, Schmieder RE, Shlyakhto E, Tsioufis C, Aboyans V, Desormais I; ESC Scientific Document Group. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J 39: 3021–3104, 2018. [Erratum in Eur Heart J 40: 475, 2019]. doi: 10.1093/eurheartj/ehy339. [DOI] [PubMed] [Google Scholar]
- 19. Lurbe E, Agabiti-Rosei E, Cruickshank JK, Dominiczak A, Erdine S, Hirth A, Invitti C, Litwin M, Mancia G, Pall D, Rascher W, Redon J, Schaefer F, Seeman T, Sinha M, Stabouli S, Webb NJ, Wühl E, Zanchetti A. 2016 European Society of Hypertension guidelines for the management of high blood pressure in children and adolescents. J Hypertens 34: 1887–1920, 2016. doi: 10.1097/HJH.0000000000001039. [DOI] [PubMed] [Google Scholar]
- 20. Agbaje AO, Barker AR, Tuomainen T-P. Cardiorespiratory fitness, fat mass, and cardiometabolic health with endothelial function, arterial elasticity, and stiffness. Med Sci Sports Exerc 54: 141–152, 2022. doi: 10.1249/mss.0000000000002757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Falaschetti E, Hingorani AD, Jones A, Charakida M, Finer N, Whincup P, Lawlor DA, Davey Smith G, Sattar N, Deanfield JE. Adiposity and cardiovascular risk factors in a large contemporary population of pre-pubertal children. Eur Heart J 31: 3063–3072, 2010. doi: 10.1093/eurheartj/ehq355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care 27: 1487–1495, 2004. doi: 10.2337/diacare.27.6.1487. [DOI] [PubMed] [Google Scholar]
- 23. Ascaso JF, Pardo S, Real JT, Lorente RI, Priego A, Carmena R. Diagnosing insulin resistance by simple quantitative methods in subjects with normal glucose metabolism. Diabetes Care 26: 3320–3325, 2003. doi: 10.2337/diacare.26.12.3320. [DOI] [PubMed] [Google Scholar]
- 24. Golding G, Pembrey P, Jones J; ALSPAC Study Team. ALSPAC – The Avon Longitudinal Study of Parents and Children I. Study methodology. Paediatr Perinat Epidemiol 15: 74–87, 2001. doi: 10.1046/j.1365-3016.2001.00325.x. [DOI] [PubMed] [Google Scholar]
- 25. Ahimastos AA, Formosa M, Dart AM, Kingwell BA. Gender differences in large artery stiffness pre- and post puberty. J Clin Endocrinol Metab 88: 5375–5380, 2003. doi: 10.1210/jc.2003-030722. [DOI] [PubMed] [Google Scholar]
- 26. Cote AT, Phillips AA, Harris KC, Sandor GGS, Panagiotopoulos C, Devlin AM. Obesity and arterial stiffness in children: Systematic review and meta-analysis. Arterioscler Thromb Vasc Biol 35: 1038–1044, 2015. doi: 10.1161/ATVBAHA.114.305062. [DOI] [PubMed] [Google Scholar]
- 27. Ryder JR, Northrop E, Rudser KD, Kelly AS, Gao Z, Khoury PR, Kimball TR, Dolan LM, Urbina EM. Accelerated early vascular aging among adolescents with obesity and/or type 2 diabetes mellitus. J Am Heart Assoc 9: e014891, 2020. doi: 10.1161/JAHA.119.014891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Agbaje AO, Barker AR, Tuomainen T-P. Cumulative muscle mass and blood pressure but not fat mass drives arterial stiffness and carotid intima-media thickness progression in the young population and is unrelated to vascular organ damage. Hypertens Res, 2022. doi: 10.1038/s41440-022-01065-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Otsuki T, Maeda S, Iemitsu M, Saito Y, Tanimura Y, Ajisaka R, Miyauchi T. Relationship between arterial stiffness and athletic training programs in young adult men. Am J Hypertens 20: 967–973, 2007. doi: 10.1016/j.amjhyper.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 30. Agbaje AO, Barmi S, Sansum KM, Baynard T, Barker AR, Tuomainen T-P. Temporal longitudinal associations of carotid-femoral pulse wave velocity and carotid intima-media thickness with resting heart rate and inflammation in youth. J Appl Physiol (1985), 2023. doi: 10.1152/japplphysiol.00701.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chung ST, Katz LEL, Stettler-Davis N, Shults J, Sherman A, Ha J, Stefanovski D, Boston RC, Rader DJ, Magge SN. The relationship between lipoproteins and insulin sensitivity in youth with obesity and abnormal glucose tolerance. J Clin Endocrinol Metab 107: 1541–1551, 2022. doi: 10.1210/clinem/dgac113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Boutouyrie P, Chowienczyk P, Humphrey JD, Mitchell GF. Arterial stiffness and cardiovascular risk in hypertension. Circ Res 128: 864–886, 2021. doi: 10.1161/CIRCRESAHA.121.318061. [DOI] [PubMed] [Google Scholar]
- 33. Yki-Järvinen H, Westerbacka J. Insulin resistance, arterial stiffness and wave reflection. Adv Cardiol 44: 252–260, 2007. doi: 10.1159/000096746. [DOI] [PubMed] [Google Scholar]
- 34. Aronson D. Cross-linking of glycated collagen in the pathogenesis of arterial and myocardial stiffening of aging and diabetes. J Hypertens 21: 3–12, 2003. doi: 10.1097/00004872-200301000-00002. [DOI] [PubMed] [Google Scholar]
- 35. Creager MA, Lüscher TF, Cosentino F, Beckman JA. Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: Part I. Circulation 108: 1527–1532, 2003. doi: 10.1161/01.CIR.0000091257.27563.32. [DOI] [PubMed] [Google Scholar]
- 36. Pahkala K, Laitinen TT, Niinikoski H, Kartiosuo N, Rovio SP, Lagström H, Loo BM, Salo P, Jokinen E, Magnussen CG, Juonala M, Simell O, Jula A, Rönnemaa T, Viikari J, Raitakari OT. Effects of 20-year infancy-onset dietary counselling on cardiometabolic risk factors in the Special Turku Coronary Risk Factor Intervention Project (STRIP): 6-year post-intervention follow-up. Lancet Child Adolesc Health 4: 359–369, 2020. doi: 10.1016/S2352-4642(20)30059-6. [DOI] [PubMed] [Google Scholar]
- 37. Agbaje AO, Lloyd-Jones DM, Magnussen CG, Tuomainen T-P. Cumulative dyslipidemia with arterial stiffness and carotid IMT progression in asymptomatic adolescents: a simulated intervention longitudinal study using temporal inverse allocation model. Atherosclerosis 364: 39–48, 2023. doi: 10.1016/j.atherosclerosis.2022.11.011. [DOI] [PubMed] [Google Scholar]
- 38. Maher GM, Ryan L, McCarthy FP, Hughes A, Park C, Fraser A, Howe LD, Kearney PM, O'Keeffe LM. Puberty timing and markers of cardiovascular structure and function at 25 years: a prospective cohort study. BMC Med 19: 78, 2021. doi: 10.1186/s12916-021-01949-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Fig. S1 and Supplemental Table S1: https://doi.org/10.6084/m9.figshare.21997502.v1.
Data Availability Statement
The informed consent obtained from ALSPAC participants does not allow the data to be made freely available through any third-party maintained public repository. However, data used for this submission can be made available on request to the ALSPAC Executive. The ALSPAC data management plan describes in detail the policy regarding data sharing, which is through a system of managed open access. Full instructions for applying for data access can be found here: http://www.bristol.ac.uk/alspac/researchers/access/. The ALSPAC study website contains details of all the data that are available (http://www.bristol.ac.uk/alspac/researchers/our-data/).