Keywords: atherosclerosis, birth cohort, carotid-femoral pulse wave velocity, causal inference, high-sensitivity C reactive protein
Abstract
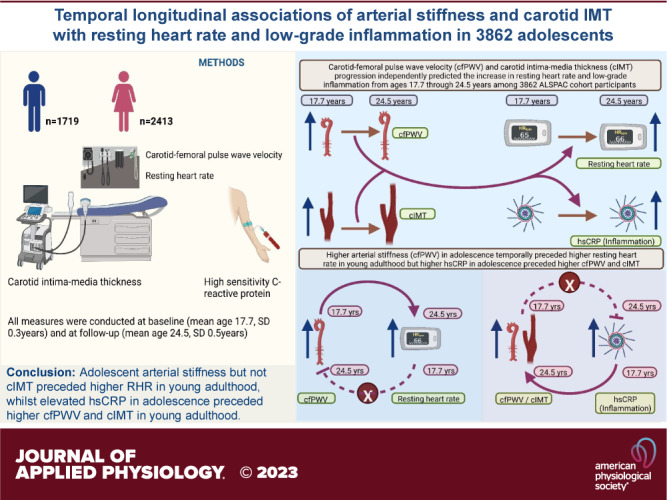
We examined the temporal longitudinal associations of carotid-femoral pulse wave velocity (cfPWV) and carotid intima-media thickness (cIMT) with the risk of elevated resting heart rate (RHR) and high-sensitivity C-reactive protein (hsCRP). We studied 3,862 adolescents, mean age 17.7 (SD 0.3 yr), followed-up for 7 yr until age 24.5 (0.7) yr, from the Avon Longitudinal Study of Parents and Children, UK. RHR, fasting plasma hsCRP, cfPWV, and cIMT were repeatedly assessed and analyzed using logistic regression, linear mixed-effect, and structural equation models adjusting for important covariates. Among 3,862 adolescents [2,143 (55.5%) female], 10% and 44% were at moderate-to-high risk of elevated RHR and hsCRP at 24.5 yr, respectively. Higher cfPWV at 17.7 yr was associated with elevated RHR risk at follow-up [odds-ratio (OR) 1.58 (CI 1.20–2.08); P = 0.001], whereas cIMT at 17.7 yr was associated with elevated hsCRP risk [OR 2.30 (1.18–4.46); P = 0.014] at follow-up, only among females. In mixed model, 7-yr progression in cfPWV was directly associated with 7-yr increase in RHR [effect-estimate 6 beats/min (1–11); P = 0.017] and hsCRP. cIMT progression was associated with 7-yr increase in RHR and hsCRP. In cross-lagged model, higher cfPWV at 17.7 yr was associated with higher RHR (β = 0.06, standard error = 3.85, P < 0.0001) at 24.5 yr but RHR at 17.7 yr was unassociated with cfPWV at 24.5 yr. Baseline cIMT or RHR was unassociated with either outcome at follow-up. Higher hsCRP at 17.7 yr was associated with higher cfPWV and cIMT at 24.5 yr. In conclusion, adolescent arterial stiffness but not cIMT appears to precede higher RHR in young adulthood, whereas elevated hsCRP in adolescence preceded higher cfPWV and cIMT.
NEW & NOTEWORTHY Higher arterial stiffness but not carotid-intima media thickness in adolescence preceded higher resting heart rate in young adulthood, however, elevated high sensitivity C-reactive protein in adolescence preceded higher arterial stiffness and carotid intima-thickness in young adulthood in the temporal causal path. Low-grade inflammation during adolescence may be causally associated with the development of subclinical arteriosclerosis and atherosclerosis in young adulthood.
INTRODUCTION
Childhood and adolescent cardiovascular risk factors have recently been associated with premature cardiovascular mortality at the age of 40 yr (1). It is of public health significance to identify the earliest time point when these risk factors alter cardiovascular structure and function. Elevated resting heart rate and inflammation measured with high-sensitivity C-reactive protein (hsCRP) are risk factors in the pathogenesis of cardiovascular diseases, including the lifelong process of atherogenesis (2–5). These risk factors have also been associated with the intermediate processes of arteriosclerosis and atherosclerosis such as arterial stiffness and carotid intima-media thickness (cIMT), in adults (3, 4, 6). Arterial stiffness and cIMT are independent predictors of future cardiovascular events and all-cause mortality (7, 8). However, whether resting heart rate and inflammation bidirectionally and temporally associate with arterial stiffness and cIMT has never been prospectively investigated among adolescents and young adults. Bidirectionality implies that the relationships of exposures with outcomes may occur in either direction with the exposures leading to outcomes and vice versa. Thus, knowledge gaps exist regarding plausible biological pathways through which these risk factors associate with arterial stiffness and cIMT in this population, among whom primary prevention of cardiovascular disease is critical (9, 10).
We examined the temporal longitudinal associations of carotid-femoral pulse wave velocity (cfPWV), a measure of arterial stiffness, and cIMT, a measure of subclinical atherosclerosis, with resting heart rate and hsCRP among 3,862 adolescents followed up for 7 yr, using data from the Avon Longitudinal Study of Parents and Children (ALSPAC) birth cohort, England, UK. We hypothesized that higher resting heart rate and hsCRP at baseline would precede increased arterial stiffness and cIMT at follow-up.
METHODS
Study Cohort
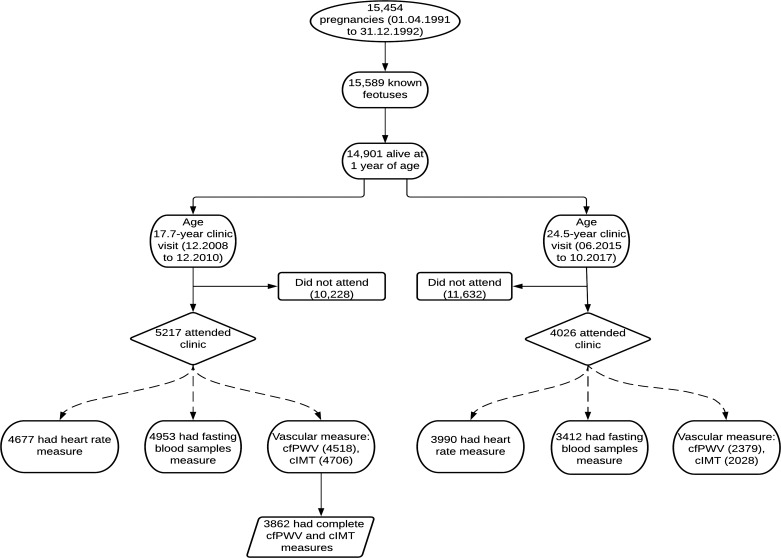
Data were from the ALSPAC birth cohort, which investigates factors that influence childhood development and growth. Altogether, 14,541 pregnancies from women residing in Avon, southwest England, UK, who had a total of 14,676 fetuses, with expected dates of delivery from April 1, 1991, to December 31, 1992 were enrolled. When the oldest children were ∼7 yr of age, an attempt was made to bolster the initial sample with eligible cases who had failed to join the study originally resulting in 913 additional pregnancies. The total sample size for analyses using any data collected after 7 yr of age is 15,454 pregnancies, resulting in 15,589 fetuses. Of these 14,901 were alive at 1 yr of age. Regular clinic visits of the children commenced at 7 yr of age and are still ongoing. Study data at 24.5 yr clinic visits that occurred from June 2015 through October 2017 were collected and managed using REDCap electronic data capture tools (11). For our analysis, we included participants who had both cfPWV and cIMT measurements at age 17.7 yr when data were collected from December 2008 through December 2010 (Fig. 1). The demographic characteristics of excluded participants were similar to those included in this study as earlier reported (12, 13). Ethical approval for the study was obtained from the ALSPAC Ethics and Law Committee and the Local Research Ethics Committees. Informed consent for the use of data collected via questionnaires and clinics was obtained from participants following the recommendations of the ALSPAC Ethics and Law Committee at the time (14–16). Consent for biological samples has been collected in accordance with the Human Tissue Act (2004). The study was conducted in accordance with the Declaration of Helsinki.
Figure 1.
Flowchart of study participants. cfPWV, carotid-femoral pulse wave velocity; cIMT, carotid intima-media thickness; DEXA, dual-energy Xray absorptiometry. Participants that had complete outcomes of interest at baseline were included in the analyses.
Anthropometry and Body Composition
Anthropometry (height and weight) at ages 17.7 and 24.5 yr was assessed using standard protocols (12, 13, 17, 18). At ages 17.7 and 24.5 yr, body composition (total fat mass and lean mass) was assessed using dual-energy X-ray absorptiometry as earlier described. We calculated body mass index by dividing weight by squared height. Participants having >24.9 kg/m2 of body mass index were classified as overweight and/or obese, whereas those below this cut point were classified as normal weight. We utilized a single cut point due to few participants at other cut-offs such as the underweight and obese category.
Vascular Phenotype
At ages 17.7 and 24.5 yr, cfPWV was computed from pressure waveforms obtained using the Vicorder device (Skidmore Medical, Bristol, UK) (12, 13). cIMT was assessed by ultrasound using a linear 12-MHz transducer (Vivid7, GE Medical, Chicago, IL), whereas at 24.5 yr, a CardioHealth Panasonic and a 13.5 MHz linear array broadband transducer (probe; center frequency 9.0 MHz) was used. Participants had between 1 and 3 cIMT measures for both the right and left carotid arteries. For our analysis, we computed the mean of the average measurement of the right and left common carotid arteries as cIMT as previously detailed (12, 13). Interobserver variability for cIMT was assessed in a separate sample of 25 young adults (coefficient of variation: 4.4 ± 2.2%).
Cardiometabolic and Lifestyle Factors
Resting heart rate and blood pressure were measured at ages 17.7 yr using an Omron 705-IT oscillometric monitor as previously detailed (12, 13). Resting heart rate and blood pressure readings at the 24.5-yr clinic visit were taken using an Omron M6 upper arm blood pressure/pulse monitor. Participants at age 24.5 yr whose resting heart rate was >80 beats/min were categorized as at moderate to high risk of elevated resting heart rate (19). Using standard protocols, fasting plasma samples at ages 17.7 and 24.5 yr were collected, spun, and frozen at –80°C and a detailed assessment of glucose, insulin, hsCRP, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, and triglycerides have been reported (all assay coefficient of variation was <5%) (12, 13, 20). Participants at age 24.5 yr with hsCRP >1.0 mg/L were categorized at moderate to high risk of elevated hsCRP (21). Before data were collected, a short questionnaire was completed to ascertain details of a recent vaccination and recent infection, and participants with positive responses were rescheduled.
Questionnaires to assess smoking behavior were administered at the 17.7-yr and 24.5-yr clinic visits. At the 17.7-yr clinic visit, participants were briefly asked about their personal and family (mother, father, and siblings) medical history such as a history of hypertension, diabetes, high cholesterol, and vascular disease (12, 13). Physical activity at age 15.5 yr was assessed with ActiGraph accelerometer worn for 7 continuous days. Moderate to vigorous physical activity cut point was >2,296 counts/min as earlier described (12, 13). At 24.5 yr, physical activity was assessed using ActiGraph GT3X+ accelerometer device worn for 4 consecutive days, ideally starting the day after the clinic visit. Moderate to vigorous physical activity reported in minutes per day was based on a previously established cutoff of >2,020 counts/min (22).
Statistical Analysis
Participants’ descriptive characteristics were summarized as means and standard deviation, medians and interquartile ranges, or frequencies and percentages. We explored sex differences using Independent Student t tests, Mann–Whitney U tests, or Chi-square tests for normally distributed, skewed, or dichotomous variables, respectively. We assessed the normality of variables by histogram curve, quantile-quantile plot, and Kolmogorov–Smirnov tests. We conducted a logarithmic and reciprocal transformation of skewed variables and confirmed normality before further analysis.
We investigated the separate longitudinal associations of cfPWV and cIMT at 17.7 yr with each of the resting heart rate and hsCRP categories at 24.5 yr using logistic regression. The logistic regression outcome variables were defined as participants with resting heart rate >80 beats/min categorized at risk of elevated resting heart rate and hsCRP >1.0 mg/L were categorized at risk of elevated hsCRP. Multivariable analysis for all participants was adjusted for sex and baseline (17.7 yr) covariates viz, age, low-density lipoprotein cholesterol, insulin, triglyceride, high-density lipoprotein cholesterol, glucose, systolic BP, fat mass, lean mass, heart rate, hsCRP, moderate to vigorous physical activity at 15.5 yr, smoking status, and family history of hypertension/diabetes/high cholesterol/vascular disease. The sex-stratified multivariable logistic regression analysis was not adjusted for sex.
We examined the separate prospective associations of the 7-yr progression in cfPWV and cIMT with the longitudinal increase in both resting heart rate and hsCRP serially measured at ages 17.7 and 24.5 yr using linear mixed-effect models with restricted maximum likelihood estimation. The estimates quantified the effect of the longitudinal progression of the predictors on the longitudinal progression of the outcome variables using raw values at baseline and follow-up (23). We decided a priori to select the model with the least Bayesian information criterion which resulted in a model with sex as a factor and a random intercept modeled on the subject level. We selected a variance component covariance type and determined the effect of the progression in predictors on progression in outcome variables. The better model for repeated measures was that which was adjusted for the difference in time (yr) between baseline and follow-up as a continuous variable rather than as categorical: baseline (time t − 1) and follow-up (time t − 2). Multivariable analyses for all participants and weight stratified models were adjusted for sex, age at 17.7 yr, time in yr between 17.7 and 24.5 yr, and covariates measured at 17.7 and 24.5 yr such as low-density lipoprotein cholesterol, insulin, triglyceride, high-density lipoprotein cholesterol, fasting plasma glucose, systolic BP, fat mass, lean mass, smoking status, family history of hypertension/diabetes/high cholesterol/vascular disease, and moderate to vigorous physical activity at 15.5 and 24.5 yr, in addition to resting heart rate and hsCRP depending on the outcome. Sex-stratified multivariable analysis was not adjusted for sex.
Finally, we used structural equation modeling with autoregressive cross-lagged design to examine the separate temporal associations of cfPWV and cIMT with both resting heart rate and hsCRP. The cross-lagged models first tested the separate associations of cfPWV and cIMT at 17.7 yr with each of resting heart rate and hsCRP at 24.5 yr; and second tested the separate associations of resting heart rate and hsCRP at 17.7 yr with cfPWV and cIMT at 24.5 yr. These models were adjusted for all the baseline covariates listed earlier, including the time in yr between 17.7 and 24.5 yr. In the cross-lagged path design, the potential association could be higher cfPWV and cIMT leading to higher resting heart rate and hsCRP, higher resting heart rate and fasting plasma hsCRP leading to cfPWV and cIMT or bidirectional associations of cfPWV and cIMT with resting heart rate and hsCRP. If a path from cfPWV and cIMT at time t − 1 (17.7 yr) to each of resting heart rate and hsCRP at time t − 2 (24.5 yr) attain statistical significance (P value < 0.05), changes in the earlier variables are considered to lead to changes in the later and vice versa. A stronger predictive effect is determined by a larger standardized regression coefficient. We concluded that the cross-lagged models had good fits with the following indices: the root-mean-square error of approximation (<0.013, the value <0.05 is considered to indicate a good model-data fit), the normed fit index (>1.000), the relative fit index (>0.976), the incremental fit index (>1.000), the Tucker–Lewis Fit Index (>0.990), the comparative fit index (>1.000), which are considered good fit if values are >0.90 (24).
All covariates were selected based on previous studies (3, 9, 12, 13, 25). We excluded pubertal status/somatic maturation from the model because all participants had reached adult-like maturity status by 17.7 yr of age (17). Height was not adjusted because of near-perfect collinearity with lean mass (r = 0.95). We performed collinearity diagnoses and accepted results with a variance inflation factor <5. We considered differences and associations with a two-sided P value <0.05 as statistically significant and made conclusions based on effect estimates and their confidence intervals (CIs) or standard errors (SEs). Missing data were accounted for using multiple imputations with 20 cycles of imputed data set, which has been efficient in the study population (12, 13, 17, 26). The imputed data set was used only in the logistic regression analyses. Extensive detail on missing data analyses of the current studied population has been reported elsewhere (12, 13). At 17.7 yr, metabolic and lipid variables had 33% missing data (12, 13, 17). We observed statistically significant sex interactions and presented sex-based results in addition to grouped results. Analyses involving 40% of a sample of 10,000 ALSPAC children at 0.8 statistical power, 0.05 α, and two-sided P value would show a minimum detectable effect size of 0.049 standard deviations if they had relevant exposure for a normally distributed quantitative variable (27). All statistical analyses were performed using SPSS statistics software, Version 27.0 (IBM Corp, Armonk, NY), and cross-lagged structural equation modeling was conducted using IBM AMOS version 27.0.
RESULTS
Cohort Study Characteristics
In the ALSPAC birth cohort, 14,901 children were alive at 1 yr of age, of whom 5,217 adolescents and 4,026 young adults participated in the 24.5-yr follow-up clinic visit, respectively (Fig. 1). We examined 3,862 participants who had complete cfPWV and cIMT measurements at age 17.7 yr. Females had a higher resting heart rate and hsCRP than males at both 17.7 and 24.5 yr. At 24.5 yr, 6% of 952 males and 12% of 1,495 females were at risk of elevated resting heart rate. Also, 35% of 752 males and 50% of 1,100 females were at risk of high hsCRP. Other participants’ characteristics are shown in Table 1.
Table 1.
Descriptive characteristics of cohort participants
| 17.7 Yr |
24.5 Yr |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Male |
Female |
Male |
Female |
|||||||
| Variables | n | Mean (SD) | n | Mean (SD) | P Value | n | Mean (SD) | n | Mean (SD) | P Value |
| Anthropometry | ||||||||||
| Age, yr | 1,719 | 17.72 (0.32) | 2,143 | 17.72 (0.34) | 0.753 | 954 | 24.58 (0.72) | 1,502 | 24.50 (0.74) | 0.008 |
| Height, m | 1,693 | 1.79 (0.07) | 2,112 | 1.65 (0.06) | <0.0001 | 949 | 1.80 (0.07) | 1,484 | 1.66 (0.06) | <0.0001 |
| *Weight, kg | 1,696 | 69.40 (14.45) | 2,114 | 60.40 (13.50) | <0.0001 | 948 | 77.80 (17.65) | 1,484 | 64.65 (17.10) | <0.0001 |
| Somatic maturation (n, %) | 1,600 | 1,601 (100) | 1,927 | 1,927 (100) | NA | NA | ||||
| Ethnicity-White (n, %) | 1,565 | 1,498 (95.7) | 1,925 | 1,848 (96.0) | 0.670 | NA | ||||
| Body composition | ||||||||||
| *Total fat mass, kg | 1,675 | 10.28 (9.81) | 2,080 | 19.24 (10.44) | <0.0001 | 927 | 17.91 (10.35) | 1,436 | 22.12 (11.91) | <0.0001 |
| Lean mass, kg | 1,675 | 54.83 (8.13) | 2,080 | 37.68 (5.16) | <0.0001 | 927 | 56.09 (10.07) | 1,436 | 40.82 (6.79) | <0.0001 |
| *Body mass index, kg/m2 | 1,693 | 21.54 (4.05) | 2,112 | 21.95 (4.42) | <0.0001 | 948 | 23.98 (4.79) | 1,483 | 23.51 (5.70) | 0.177 |
| Metabolic profile | ||||||||||
| Total cholesterol, mmol/L | 1,276 | 3.56 (0.62) | 1,310 | 3.94 (0.69) | <0.0001 | 839 | 4.34 (0.85) | 1,174 | 4.48 (0.82) | <0.0001 |
| High-density lipoprotein, mmol/L | 1,276 | 1.19 (0.26) | 1,310 | 1.35 (0.32) | <0.0001 | 839 | 1.40 (0.37) | 1,174 | 1.66 (0.42) | <0.0001 |
| Low-density lipoprotein, mmol/L | 1,276 | 2.00 (0.56) | 1,310 | 2.21 (0.63) | <0.0001 | 837 | 2.46 (0.79) | 1,174 | 2.40 (0.74) | 0.071 |
| *Triglyceride, mmol/L | 1,276 | 0.74 (0.34) | 1,310 | 0.76 (0.37) | 0.096 | 838 | 0.87 (0.55) | 1,174 | 0.81 (0.47) | <0.0001 |
| Glucose, mmol/L | 1,276 | 5.16 (0.69) | 1,310 | 4.91 (0.51) | <0.0001 | 839 | 5.46 (0.58) | 1,174 | 5.22 (0.63) | <0.0001 |
| *Insulin, mU/L | 1,258 | 6.05 (4.19) | 1,285 | 7.33 (4.37) | <0.0001 | 839 | 6.92 (4.94) | 1,174 | 7.75 (5.56) | <0.0001 |
| *High sensitivity C-reactive protein, mg/L | 1,276 | 0.44 (0.68) | 1,310 | 0.67 (1.28) | <0.0001 | 752 | 0.63 (1.10) | 1,100 | 0.99 (2.13) | <0.0001 |
| At risk of elevated hs C-reactive protein >1.0 mg/L (n, %) | NA | 752 | 264 (35) | 1,100 | 549 (50) | NA | ||||
| Vascular measures | ||||||||||
| Heart rate, beats/min | 1,717 | 63 (9) | 2,138 | 68 (10) | <0.0001 | 952 | 64 (10) | 1,495 | 69 (10) | <0.0001 |
| At risk of elevated heart rate >80 beats/min (n, %) | NA | 952 | 58 (6) | 1,495 | 177 (12) | NA | ||||
| Systolic blood pressure, mmHg | 1,717 | 120 (9) | 2,138 | 110 (8) | <0.0001 | 952 | 122 (10) | 1,495 | 112 (10) | <0.0001 |
| Diastolic blood pressure, mmHg | 1,717 | 63 (6) | 2,138 | 65 (6) | <0.0001 | 952 | 67 (8) | 1,495 | 66 (8) | 0.004 |
| *Carotid-femoral pulse wave velocity, m/s | 1,719 | 6.03 (0.70) | 2,143 | 5.54 (0.62) | <0.0001 | 640 | 6.47 (1.21) | 1,012 | 5.90 (1.03) | <0.0001 |
| *Carotid intima-media thickness, mm | 1,719 | 0.48 (0.05) | 2,143 | 0.47 (0.04) | <0.0001 | 532 | 0.54 (0.09) | 866 | 0.53 (0.08) | <0.0001 |
| Lifestyle factors | ||||||||||
| Smoked cigarettes in the past 30 days (n, %) | 1,491 | 369 (21.5) | 1,853 | 542 (25.3) | 0.004 | 674 | 171 (25.4) | 1,105 | 284 (25.7) | 0.911 |
| MVPA, min/day | 691 | 56 (31) | 884 | 40 (23) | <0.0001 | 220 | 46 (43) | 411 | 42 (35) | 0.030 |
| Family history of H-D-C-V (n, %) | 1,718 | 492 (28.6) | 2,140 | 670 (31.3) | 0.072 | NA | ||||
The values are means (standard deviations) and *median (interquartile range) except for categorical variables in frequency and percentage. n is the number of cohort participants. Differences between sexes were tested using Student’s t test for normally distributed continuous variables, Mann–Whitney U test for skewed continuous variables, and Chi-square test for dichotomous variable. A two-sided P value <0.05 is considered statistically significant. H-D-C-V, hypertension/diabetes/high cholesterol/vascular disease; hs, high sensitivity; MVPA, moderate to vigorous physical activity; NA, not available/applicable. P value for sex difference.
Longitudinal Associations of cfPWV and cIMT at 17.7 Yr with Risk Categories of Elevated Resting Heart Rate and hsCRP at Age 24.5 Yr
A higher cfPWV at 17.7 yr was associated with the risk of elevated resting heart rate at age 24.5 yr among females [odds ratio (OR) 1.58 (95% CI 1.20–2.08); P = 0.001] and among combined groups but not in males, after adjusting for cardiometabolic and lifestyle factors (Table 2). A higher cIMT at 17.7 yr was associated with the risk of elevated hsCRP at 24.5 yr among females only [OR 2.23 (CI 1.18–4.46); P = 0.014].
Table 2.
Longitudinal associations of arterial stiffness and carotid intima-media thickness at 17.7 yr with the risk of elevated resting heart rate and C-reactive protein at 24.5 yr
| Elevated Resting Heart Rate, n = 377 |
Elevated Hs C-Reactive Protein, n = 1,704 |
|||
|---|---|---|---|---|
| Odds Ratio (95% CI) | P Value | Odds Ratio (95% CI) | P Value | |
| 3,862 participants (All) | ||||
| Carotid-femoral pulse wave velocity | 1.32 (1.06–1.65) | 0.014 | 1.14 (0.95–1.37) | 0.155 |
| Carotid intima-media thickness | 1.78 (0.79–4.04) | 0.165 | 1.25 (0.75–2.08) | 0.383 |
| 1,719 Male participants | (n = 112) | (n = 614) | ||
| Carotid-femoral pulse wave velocity | 1.14 (0.71–1.83) | 0.592 | 1.15 (0.92–1.45) | 0.219 |
| Carotid intima-media thickness | 0.57 (0.14–2.34) | 0.428 | 0.58 (0.23–1.45) | 0.236 |
| 2,413 Female participants | (n = 265) | (n = 1,071) | ||
| Carotid-femoral pulse wave velocity | 1.58 (1.20–2.08) | 0.001 | 1.01 (0.81–1.26) | 0.943 |
| Carotid intima-media thickness | 2.45 (0.91–6.57) | 0.075 | 2.30 (1.18–4.46) | 0.014 |
Multivariable model was adjusted for sex only in all participants, age at 17.7 yr, time in years between 17.7 and 24.5 yr, and covariates at 17.7 yr such as heart rate or high-sensitivity C-reactive protein depending on the outcome, fasting low-density lipoprotein cholesterol, insulin, triglyceride, high-density lipoprotein cholesterol, glucose, systolic blood pressure, total fat mass, lean mass, moderate to vigorous physical activity at 15.5 yr, smoking status at 17.7 yr, and family history of hypertension/diabetes/high cholesterol/vascular disease. Odds ratios were from logistic regression model analyses. n is the number of cohort participants. CI, confidence interval; n, sample size of the studied population who had an elevated risk of resting heart rate (10% of 3,862 participants) and high sensitivity (hs) C-reactive protein (44% of 3,862 participants). Multiple imputations were used to account for missing cases. P value <0.05 was considered statistically significant. The risk of elevated high sensitivity C-reactive protein was classified as >1.0 mg/L and elevated resting heart rate was categorized as > 80 beats/min.
Effect of cfPWV and cIMT Progression on Increased Resting Heart Rate and hsCRP from Ages 17.7–24.5 Yr
The 7-yr cfPWV progression was directly and independently associated with the 7-yr increase in resting heart rate: [effect estimate 6 beats/min (CI 1–11); P = 0.017] and hsCRP: [0.39 mg/L (CI 0.08–0.69); P = 0.014], after full adjustment for covariates (Table 3). In the sex-stratified results, cfPWV progression was associated with resting heart rate among females but not in males (Table 3). According to body mass index-weight categories, cfPWV progression was associated with the increase in hsCRP only in normal-weight participants.
Table 3.
Effect of arterial stiffness and carotid intima-media thickness progression on resting heart rate and high sensitivity C-reactive protein progression from ages 17.7 through 24.5 yr for all participants, sex-stratified, and weight stratified
| Resting Heart Rate, beats/min |
Hs C-Reactive Protein, mg/L |
|||
|---|---|---|---|---|
| Effect Estimate (95% CI) | P Value | Effect Estimate (95% CI) | P Value | |
| All participants, n = 3,862 | ||||
| Carotid-femoral pulse wave velocity | 6.008 (1.106–10.910) | 0.017 | 0.383 (0.080–0.686) | 0.014 |
| Carotid intima-media thickness | 1.185 (0.601–1.769) | <0.0001 | 0.085 (0.032–0.138) | 0.002 |
| Male participants, n = 1,719 | ||||
| Carotid-femoral pulse wave velocity | 0.515 (−7.591 to 8.621) | 0.899 | 0.323 (−0.013 to 0.660) | 0.059 |
| Carotid intima-media thickness | 0.321 (−0.682 to 1.325) | 0.526 | 0.089 (0.020–0.158) | 0.012 |
| Female participants, n = 2,413 | ||||
| Carotid-femoral pulse wave velocity | 8.743 (2.230–15.256) | 0.009 | 0.329 (−0.118 to 0.775) | 0.146 |
| Carotid intima-media thickness | 1.732 (1.022– 2.443) | <0.0001 | 0.105 (0.015–0.195) | 0.024 |
| Normal weight participants, <24.99 kg/m2 (n = 3,038) | ||||
| Carotid-femoral pulse wave velocity | 1.640 (−4.167 to 7.448) | 0.580 | 0.370 (0.015–0.725) | 0.041 |
| Carotid intima-media thickness | 0.765 (−0.232 to 1.761) | 0.131 | 0.037 (−0.013 to 0.086) | 0.143 |
| Overweight and obese participants, >24.99 kg/m2 (n = 767) | ||||
| Carotid-femoral pulse wave velocity | −7.461 (−21.369 to 0.645) | 0.292 | 0.372 (−0.393 to 1.137) | 0.336 |
| Carotid intima-media thickness | 1.352 (−0.987 to 3.691) | 0.253 | 0.049 (−0.071 to 0.170) | 0.420 |
Multivariable analyses were adjusted for sex only in all participants and weight stratified models, time in years between 17.7 and 24.5 yr, age at 17.7 yr, and covariates at 17.7 and 24.5 yr such as fasting low-density lipoprotein cholesterol, insulin, triglyceride, high-density lipoprotein cholesterol, glucose, systolic blood pressure, total fat mass, lean mass, moderate to vigorous physical activity at 15.5 and 24.5 yr, smoking status at 17.7 and 24.5 yr, and family history of hypertension/diabetes/high cholesterol/vascular disease, in addition to heart rate or high-sensitivity C-reactive protein depending on the outcome. Skewed variables were logarithmically transformed before analyses. Effect estimate was from linear mixed-effect models which estimate mean difference from baseline to follow-up. n is the number of cohort participants. CI, confidence interval; hs, high-sensitivity. P value <0.05 was considered statistically significant.
The 7-yr cIMT progression was associated with the 7-yr increase in resting heart rate: [1 beat/min (CI 1–2); P < 0.0001] and hsCRP: [0.09 mg/L (CI 0.03–0.14); P = 0.002] after full covariate adjustment (Table 3). These findings were consistent among females but among males cIMT progression was associated with increased hsCRP only. According to body mass index-weight categories, cIMT progression had no statistically significant associations with both resting heart rate and hsCRP in either normal weight or overweight/obese participants.
Cross-Lagged Temporal Relationships of cfPWV and cIMT with Resting Heart Rate and hsCRP
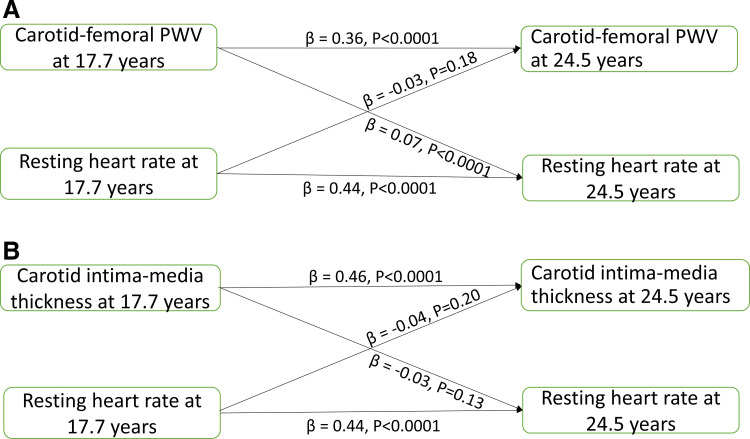
Resting heart rate, hsCRP, cfPWV, and cIMT at 17.7 yr were directly associated with their respective individual variables at 24.5 yr (Table 4). Higher cfPWV at 17.7 yr was associated with higher resting heart rate (standardized regression coefficient (β) = 0.07, P < 0.0001) at 24.5 yr, but resting heart rate at 17.7 yr was not associated with cfPWV at 24.5 yr, after adjustment for baseline covariates (Fig. 2A and Table 4). Higher cfPWV at 17.7 yr was associated with higher resting heart rate at 24.5 yr among females but not in males (β = 0.12, P < 0.0001), but resting heart rate at 17.7 yr was not associated with cfPWV at 24.5 yr in males and females (Table 4). cfPWV at 17.7 yr was not associated with hsCRP at 24.5 yr, but higher hsCRP at 17.7 yr was associated with higher cfPWV at 24.5 yr in the total cohort (β = 0.06, P = 0.037) and among females (Table 4).
Table 4.
Auto-regressive cross-lagged temporal analyses of carotid-femoral pulse wave velocity and carotid intima-media thickness with resting heart rate and high sensitivity C-reactive protein at 17.7 and 24.5 yr of age
| Auto-Regressive | B | β | SE | P Value |
|---|---|---|---|---|
| 3,862 Participants | ||||
| cfPWV T1 |
0.496 | 0.356 | 0.033 | <0.0001 |
| cIMT T1 |
0.501 | 0.459 | 0.026 | <0.0001 |
| Heart rate T1 |
0.454 | 0.438 | 0.020 | <0.0001 |
| hsCRP T1 |
0.231 | 0.218 | 0.028 | <0.0001 |
| Cross lagged | ||||
| cfPWV T1 |
13.293 | 0.067 | 3.852 | <0.0001 |
| Heart rate T1 |
<0.0001 | −0.032 | <0.0001 | 0.179 |
| cfPWV T1 |
0.231 | 0.023 | 0.243 | 0.341 |
| hsCRP T1 |
0.008 | 0.058 | 0.004 | 0.037 |
| cIMT T1 |
−0.029 | −0.039 | 0.022 | 0.196 |
| Heart rate T1 |
−0.059 | −0.027 | 0.039 | 0.129 |
| cIMT T1 |
−0.181 | −0.014 | 0.284 | 0.523 |
| hsCRP T1 |
0.009 | 0.062 | 0.004 | 0.035 |
| 1,719 Male participants | ||||
| Cross lagged | ||||
| cfPWV T1 |
−1.195 | −0.006 | 6.050 | 0.843 |
| Heart rate T1 |
<0.0001 | −0.040 | <0.0001 | 0.294 |
| cfPWV T1 |
0.561 | 0.058 | 0.341 | 0.100 |
| hsCRP T1 |
0.004 | 0.028 | 0.007 | 0.518 |
| cIMT T1 |
5.361 | 0.022 | 7.124 | 0.452 |
| Heart rate T1 |
0.000 | −0.034 | 0.000 | 0.398 |
| cIMT T1 |
0.375 | 0.033 | 0.402 | 0.352 |
| hsCRP T1 |
0.006 | 0.055 | 0.003 | 0.015 |
| 2,413 Female participants | ||||
| Cross lagged | ||||
| cfPWV T1 |
24.701 | 0.119 | 5.025 | <0.0001 |
| Heart rate T1 |
0.000 | −0.034 | 0.000 | 0.265 |
| cfPWV T1 |
0.002 | 0.000 | 0.339 | 0.995 |
| hsCRP T1 |
0.010 | 0.083 | 0.002 | <0.0001 |
| cIMT T1 |
−14.249 | −0.058 | 5.906 | 0.016 |
| Heart rate T1 |
0.000 | −0.019 | 0.000 | 0.555 |
| cIMT T1 |
−0.587 | −0.044 | 0.396 | 0.138 |
| hsCRP T1 |
0.011 | 0.065 | 0.006 | 0.058 |
B, unstandardized regression; β, standardized regression, SE, standard error; cfPWV, carotid-femoral pulse wave velocity; cIMT, carotid intima-media thickness; hscrp, high sensitivity C-reactive protein; time T1, 17.7 yr of age; time T2, 24.5 yr. Autoregressive cross-lagged analyses were adjusted for sex, time in years between ages 17.7 and 24.5 yr, and other covariates at 17.7 yr such as age, fasting low-density lipoprotein cholesterol, insulin, triglyceride, high-density lipoprotein cholesterol, glucose, systolic blood pressure, total fat mass, lean mass, moderate to vigorous physical activity at 15.5 yr, smoking status and family history of hypertension/diabetes/high cholesterol/vascular disease in addition to high-sensitivity C-reactive protein or heart rate depending on the outcome. Skewed variables were logarithmically transformed before analyses. Sex-based analyses were not adjusted for sex.
Figure 2.
Cross-lagged temporal longitudinal associations of arterial stiffness (A) and carotid intima-media thickness (B) with resting heart rate. Autoregressive cross-lagged analyses were adjusted for sex, time in years between ages 17.7 and 24.5 yr, and other covariates at 17.7 yr viz; age, systolic blood pressure, total fat mass, lean mass, moderate to vigorous physical activity at 15.5 yr, smoking status, family history of hypertension/diabetes/high cholesterol/vascular disease in addition to fasting plasma samples, namely, low-density lipoprotein cholesterol, insulin, triglyceride, high-sensitivity C-reactive protein, high-density lipoprotein cholesterol, and glucose. PWV, pulse wave velocity.
Higher cIMT at 17.7 yr was associated with lower resting heart rate at 24.5 yr in females, but not in males and the total cohort, just as resting heart rate at 17.7 yr was not associated with cIMT at 24.5 yr in the total cohort, males, and females (Fig. 2B and Table 4). cIMT at 17.7 yr was not associated with hsCRP at 24.5 yr in the total cohort, males, and females, but higher hsCRP at 17.7 yr was associated with higher cIMT at 24.5 yr, in the total cohort (β = 0.06, P = 0.035) and in males (Table 4).
DISCUSSION
In a very large cohort of adolescents, we present novel temporal longitudinal findings where arterial stiffness and carotid thickness are potential predictors of elevated resting heart rate and low-grade inflammation, independent of cardiometabolic and lifestyle factors. First, we observed that higher cfPWV at 17.7 yr was associated with the risk of elevated resting heart rate at 24.5 yr, albeit only among females, whereas higher cIMT at 17.7 yr was associated with the risk of higher hsCRP among females at 24.5 yr. Next, we reported that cfPWV and cIMT progression were directly associated with the 7-yr increase in resting heart rate and hsCRP, particularly among females. Finally, using cross-lagged structural equation models, higher cfPWV at 17.7 yr temporally preceded higher resting heart rate at 24.5 yr. However, higher hsCRP at 17.7 yr preceded higher cfPWV and cIMT at 24.5 yr. The temporal findings appear to be dependent on sex and the risk factor because effects were consistently stronger in females, and hsCRP preceded arterial outcomes, whereas arterial outcomes preceded resting heart rate.
Arterial Stiffness with the Risk of Elevated Resting Heart Rate and hsCRP
Epidemiological reports among adults have been divergent on the effects of resting heart rate on arterial stiffness and mechanistic explanation of the relationship between changes in cfPWV and heart rate remain inconclusive (3, 4). Recently a 5-yr longitudinal study among 448 adolescents with a mean age of 17.6 yr found that baseline resting heart rate was not associated with the change in cfPWV (9). Traditionally, risk factors like resting heart rate are examined as predictors of altered arterial outcomes such as cfPWV (9), but this may be grossly inadequate in understanding biological mechanisms, especially in a healthy young population. It remains unknown whether resting heart rate is a precursor or consequence of arterial stiffness, particularly among adolescents. We observed that adolescents’ arterial stiffness predicted the risk of elevated resting heart rate in young adulthood and that arterial stiffness progression was directly associated with the 7-yr increase in resting heart rate. Using a powerful cross-lagged autoregressive statistical tool to untangle temporal and independent longitudinal associations, we found that higher arterial stiffness during adolescence preceded a higher resting heart rate in young adulthood. However, adolescents resting heart rate did not precede cfPWV in young adulthood in line with a previous study (9).
A novel aspect of our findings is that the temporal longitudinal association of arterial stiffness and resting heart rate was strongly consistent among females but not in males, irrespective of the statistical modeling approach. A plausible explanation could be that females had higher resting heart rates, smoked more, and were less physically active in comparison with males. A higher heart rate among females in comparison with males has been reported (4, 28). Repetitive stretching induces fatigue of the arterial wall and increased stiffness via a viscoelastic effect due to wall composition may be induced by increased heart rate (3, 4). Smoking and decreased physical activity have been independently associated with a significant increase in heart rate (29, 30). Nonetheless, controlling for these risk factors did not alter the results. Hence, the only explanation for the sex difference could be inherent biological differences.
Elevated heart rate and blood pressure may elicit a cascade of increased sympathetic modulation, vascular shear stress, endothelial damage, and arterial wall fatigue-inducing repetitive stretching, which may accelerate atherosclerosis (3, 4, 6, 31). A cross-sectional study among 347 participants, which included an intervention study among 9–12 participants, all aged 19–29 yr, concluded that the autonomic nervous system does not directly regulate aortic stiffness, but aortic stiffness changed in response to changes in heart rate and mean arterial pressure (32). This study further noted that reducing parasympathetic modulation effectively controlled blood pressure leading to a secondary increase in aortic PWV (32). However, we now provide clearer temporal longitudinal evidence in >3,800 adolescents that higher arterial stiffness may precede higher resting heart rate in young adulthood leading to a progressive arterial stiffness-elevated resting heart rate cycle, independent of the increase in blood pressure, smoking status, lipids, metabolic state, physical activity, and low-grade inflammation. The temporal longitudinal path appears unaffected by overweight/obesity status, likely because <20% of adolescents were overweight/obese.
We observed that higher hsCRP in adolescence (at baseline) preceded higher arterial stiffness in young adulthood (at follow-up) in the total cohort and among females but not vice versa and that the 7-yr arterial stiffness progression was directly associated with the 7-yr increase in hsCRP. However, among 427 adults aged 47 yr, higher hsCRP was cross sectionally associated with higher arterial stiffness (5), whereas in a study involving 458 adolescents, baseline hsCRP was not associated with a 5-yr longitudinal change in arterial stiffness (9). Our findings suggest that inflammatory processes may initiate arterial stiffening independent of lipid and metabolic alteration and that higher arterial stiffness, in turn, may lead to further inflammation. This positive feedback loop may result in arteriosclerotic and atherosclerotic disease in later life via an imbalance in arterial wall elastin and collagen ratio, if not interrupted (3, 5, 6). It is known that mechanoregulation of the extracellular matrix is fundamental to tissue homeostasis and structural adaptation to arterial wall changes, however, persistent inflammation could prevent homeostasis and drive arterial stiffening (3). This emphasizes the importance of preventing inflammation from adolescence. Inflammation temporarily preceded arterial stiffness in females but not in males, however females had a lower level of arterial stiffness compared with males. Increased inflammation in females has been related to increased adiposity but female sex-hormones such as estrogen might likely dampen the effect of low-grade inflammation on the arterial wall (33–35). Nonetheless, further experimental and mechanistic studies are warranted to explain the sex differences.
Carotid Intima-Media Thickness and Risk of Elevated Resting Heart Rate and hsCRP
A formal demonstration of the independent predictive value of altered arterial function and structure is lacking possibly due to strong covariance with blood pressure and heart rate, which are risk factors for altered arterial function and structure (3). A longitudinal study showed that adolescents’ baseline heart rate was not associated with the 5-yr change in cIMT (9). In this present study with a population approximately nine times larger than the previous study (9), we also observed that higher resting heart rate at baseline was not associated with higher cIMT in young adulthood in the reverse temporal path. Similarly, adolescent cIMT did not precede higher resting heart rate in young adulthood nor predict an increased risk of elevated resting heart rate, suggesting a lack of bidirectionality. However, the 7-yr cIMT progression was independently and directly associated with the 7-yr increase in resting heart rate. This positive finding may be due to a more robust statistical analytic method, i.e., linear mixed-effect model (23) that used raw values at both baseline and follow-up rather than a generalized linear model in which follow-up values are subtracted from baseline to derive delta-change variables as used in the previous study (9). Moreover, in the cross-lagged analysis, the standardized effect size of the baseline cIMT in association with the follow-up resting heart rate was twofold smaller than the relationship of baseline cfPWV with follow-up resting heart rate. This suggests that measures of arterial function (cfPWV) may be twice more sensitive to alteration in resting heart rate than measures of arterial structure (cIMT) (31); hence, the null association in the logistic regression and cross-lagged temporal models. Taken together, we propose that arterial stiffness rather than cIMT may help detect early alteration in resting heart rate among adolescents and young adults, akin to arterial stiffness already established as an independent predictor of future cardiovascular events and all-cause mortality (7, 31). Among females, we observed that higher cIMT at baseline may temporarily precede lower heart rate at follow-up. This could relate to physiologic vascular adaptation to the inherent higher heart rate in females compared with males.
The potential causal role of hsCRP in the long-term development of cardiovascular disease and its precursors remains unresolved (2, 9, 10). In the present study, with repeated measures, we observed that cIMT progression, a precursor of cardiovascular events (8), was directly associated with the 7-yr increase in hsCRP in both males and females, independent of the increase in lipids, fat mass, heart rate, glucose, blood pressure, smoking, and other risk factors. Besides, it appears that higher hsCRP in adolescence independently preceded higher cIMT in young adulthood. Our results contrasted a previous study involving 1,617 participants, aged 3 to 18 yr at baseline who were reexamined at ages 24 to 39 yr that concluded hsCRP measured in childhood was not associated with adult carotid IMT (36). The disparity in our findings may be that the authors analyzed hsCRP samples stored for 25 yr (32), whereas we used samples analyzed soon after clinic visits. The structural equation cross-lagged path model (10) utilized in our study improves our understanding of the potential independent mechanisms of risk imparted by subclinical or low-grade inflammation, and the likelihood of an existing vicious cycle between hsCRP and cIMT from adolescence. Females had consistently higher hsCRP levels compared with males across the observation period. The sex differences in inflammatory activity may relate to differences in visceral and subcutaneous fat, a strong determinant of hsCRP levels, or to differences in estrogen, which has been associated with higher hsCRP levels (33, 34). Therefore, an isolated hsCRP and/or cIMT measure may not be optimal for identifying individuals at risk of inflammation and subclinical atherosclerosis (2, 9, 10, 18). Given this evidence for the role of inflammation in carotid thickening and arterial stiffening in adolescence, anti-inflammatory interventions may be effective in interrupting the vicious cycle of inflammation and altered arterial function and structure (3). It was recently observed that a proinflammatory diet during childhood was associated with worsening cardiometabolic health in late adolescence and early adulthood, therefore diet rich in anti-inflammatory properties such as fruits and vegetables should be promoted in youth (37).
Strengths and Limitations
Accessible repeated gold-standard measures from an extensively phenotyped large birth cohort (ALSPAC) enabled an attempt at possible temporal longitudinal associations of cfPWV and cIMT with the risk of elevated resting heart rate and hsCRP. In addition, by applying advanced statistical tools such as cross-lagged structural equation models our findings improve understanding of asymptomatic and subclinical paths in atherosclerotic disease development. We also controlled for an extensive array of objectively measured covariates such as physical activity and body composition. However, dietary data were unavailable at ages 17.7 and 24.5 yr, but we controlled for participants’ body composition that may reflect participants’ diet (38) since proinflammatory diet has been associated with worse cardiometabolic status (37). Our adolescents were mainly Caucasian from southwest England; hence, these findings may not be generalizable to other ethnicities. An observational study design alone may be insufficient in establishing causal inference. We cannot exclude the possibility of residual bias introduced from different equipment used at different time points, such as the cIMT measure (39), lack of central hemodynamic measures, such as central BP and stroke volume, measures of physical activity only available at a mean age of 15.5 yr, and the lack of hemoglobin concentrations.
Conclusions
To our knowledge, for the first time, we showed in 3,862 adolescents that arterial stiffness in adolescence preceded elevated resting heart rate in young adulthood, and higher inflammation in adolescence preceded both higher arterial stiffness and carotid thickness in young adulthood. The 7-yr progression of arterial stiffness and cIMT were directly associated with the 7-yr increase in resting heart rate and inflammation. The temporal nature of our findings seems to depend on sex, risk factors (resting heart rate or inflammation), and arterial measures. Overall, the temporal longitudinal evidence provides potential underlying mechanisms in which arterial stiffening appears to kick-start an arterial stiffness-elevated resting heart rate vicious loop, just as inflammation seems to initiate an arterial stiffness-inflammation and cIMT-inflammation cycle. Our findings support the clinical use of arterial stiffness measures in predicting long-term alteration in cardiometabolic risk factors among adolescents (12, 13, 25, 40, 41). Further longitudinal studies are needed to replicate these temporal findings in a multi-ethnic adolescent population.
DATA AVAILABILITY
The informed consent obtained from ALSPAC participants does not allow the data to be made freely available through any third-party maintained public repository. However, data used for this submission can be made available on request to the ALSPAC Executive. The ALSPAC data management plan describes in detail the policy regarding data sharing, which is through a system of managed open access. Full instructions for applying for data access can be found here: http://www.bristol.ac.uk/alspac/researchers/access/. The ALSPAC study website contains details of all the data that are available (http://www.bristol.ac.uk/alspac/researchers/our-data/).
GRANTS
The UK Medical Research Council and Wellcome Grant Ref. Nos. 217065/Z/19/Z, 076467/Z/05/Z and the University of Bristol provide core support for ALSPAC (Avon Longitudinal Study of Parents and Children). The British Heart Foundation Grant CS/15/6/31468 funded blood pressure, carotid intima-media thickness, carotid-femoral pulse wave velocity, and Actigraph activity monitoring device measurement at 24-yr clinic visit. The Medical Research Council Grant MR/M006727/1 supported smoking data collection. A comprehensive list of grants funding is available on the ALSPAC website (http://www.bristol.ac.uk/alspac/external/documents/grant-acknowledgements.pdf); Dr. Agbaje’s research group, UndeRstanding FITness, and Cardiometabolic Health In Little Darlings (urFIT-child; https://uefconnect.uef.fi/en/group/understanding-fitness-and-cardiometabolic-health-in-little-darlings-urfit-child/), was specifically funded by the Jenny and Antti Wihuri Foundation Grant No. 00180006; the North Savo regional and central Finnish Cultural Foundation Grant Nos. 65191835 and 00200150; the Orion Research Foundation sr; the Aarne Koskelo Foundation; the Antti and Tyyne Soininen Foundation; the Paulo Foundation; the Paavo Nurmi Foundation; the Yrjö Jahnsson Foundation Grant No. 20217390; and the Finnish Foundation for Cardiovascular Research Grant No. 220021. Graphical abstract was created with BioRender.com.
DISCLAIMERS
The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.O.A. conceived and designed research; A.O.A. analyzed data; A.O.A., S.B., K.M.S., and T.B. interpreted results of experiments; A.O.A. prepared figures; A.O.A. drafted manuscript; A.O.A., S.B., K.M.S., T.B., A.R.B. and T.-P.T. edited and revised manuscript; A.O.A., S.B., K.M.S., T.B., A.R.B., and T.-P.T. approved final version of manuscript.
ACKNOWLEDGMENTS
We are extremely grateful to all the families who took part in this study, the midwives for help in recruiting them, and the whole ALSPAC team, which includes interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, receptionists, and nurses.
REFERENCES
- 1. Jacobs DR, Woo JG, Sinaiko AR, Daniels SR, Ikonen J, Juonala M, Kartiosuo N, Lehtimäki T, Magnussen CG, Viikari JSA, Zhang N, Bazzano LA, Burns TL, Prineas RJ, Steinberger J, Urbina EM, Venn AJ, Raitakari OT, Dwyer T. Childhood cardiovascular risk factors and adult cardiovascular events. N Engl J Med 386: 1877–1888, 2022. doi: 10.1056/NEJMoa2109191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Danesh J, Pepys MB. C-reactive protein and coronary disease: is there a causal link? Circulation 120: 2036–2039, 2009. doi: 10.1161/CIRCULATIONAHA.109.907212. [DOI] [PubMed] [Google Scholar]
- 3. Boutouyrie P, Chowienczyk P, Humphrey JD, Mitchell GF. Arterial stiffness and cardiovascular risk in hypertension. Circ Res 128: 864–886, 2021. doi: 10.1161/CIRCRESAHA.121.318061. [DOI] [PubMed] [Google Scholar]
- 4. Tan I, Butlin M, Spronck B, Xiao H, Avolio A. Effect of heart rate on arterial stiffness as assessed by pulse wave velocity. Curr Hypertens Rev 14: 107–122, 2018. doi: 10.2174/1573402113666170724100418. [DOI] [PubMed] [Google Scholar]
- 5. Yasmin, McEniery CM, Wallace S, Mackenzie IS, Cockcroft JR, Wilkinson IB. C-reactive protein is associated with arterial stiffness in apparently healthy individuals. Arterioscler Thromb Vasc Biol 24: 969–974, 2004. doi: 10.1161/01.ATV.zhq0504.0173. [DOI] [PubMed] [Google Scholar]
- 6. Zieman SJ, Melenovsky V, Kass DA. Mechanisms, pathophysiology, and therapy of arterial stiffness. Arterioscler Thromb Vasc Biol 25: 932–943, 2005. doi: 10.1161/01.ATV.0000160548.78317.29. [DOI] [PubMed] [Google Scholar]
- 7. Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness. A systematic review and meta-analysis. J Am Coll Cardiol 55: 1318–1327, 2010. doi: 10.1016/j.jacc.2009.10.061. [DOI] [PubMed] [Google Scholar]
- 8. Polak JF, Pencina MJ, Pencina KM, O'Donnell CJ, Wolf PA, D'Agostino RB. Carotid-wall intima—media thickness and cardiovascular events. N Engl J Med 365: 213–221, 2011. doi: 10.1056/NEJMoa1012592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ryder JR, Northrop E, Rudser KD, Kelly AS, Gao Z, Khoury PR, Kimball TR, Dolan LM, Urbina EM. Accelerated early vascular aging among adolescents with obesity and/or type 2 diabetes mellitus. J Am Heart Assoc 9: e014891, 2020. doi: 10.1161/JAHA.119.014891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gao Z, Khoury PR, McCoy CE, Shah AS, Kimball TR, Dolan LM, Urbina EM. Adiposity has no direct effect on carotid intima-media thickness in adolescents and young adults: use of structural equation modeling to elucidate indirect & direct pathways. Atherosclerosis 246: 29–35, 2016. doi: 10.1016/j.atherosclerosis.2015.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O'Neal L, McLeod L, Delacqua G, Delacqua F, Kirby J, Duda SN; REDCap Consortium. The REDCap consortium: building an international community of software platform partners. J Biomed Inform 95: 103208, 2019. doi: 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Agbaje AO, Barker AR, Tuomainen T-P. Effects of arterial stiffness and carotid intima-media thickness progression on the risk of overweight/obesity and elevated blood pressure/hypertension: a cross-lagged cohort study. Hypertension 79: 159–169, 2022. doi: 10.1161/HYPERTENSIONAHA.121.18449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Agbaje AO, Barker AR, Mitchell GF, Tuomainen T-P. Effect of arterial stiffness and carotid intima-media thickness progression on the risk of dysglycemia, insulin resistance, and dyslipidemia: a temporal causal longitudinal study. Hypertension 79: 667–678, 2022. doi: 10.1161/HYPERTENSIONAHA.121.18754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Northstone K, Lewcock M, Groom A, Boyd A, Macleod J, Timpson N, Wells N. The Avon Longitudinal Study of Parents and Children (ALSPAC): an update on the enrolled sample of index children in 2019. Wellcome Open Res 4: 51, 2019. doi: 10.12688/wellcomeopenres.15132.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fraser A, Macdonald-Wallis C, Tilling K, Boyd A, Golding J, Davey Smith G, Henderson J, Macleod J, Molloy L, Ness A, Ring S, Nelson SM, Lawlor DA. Cohort profile: the Avon Longitudinal Study of Parents and Children: ALSPAC mothers cohort. Int J Epidemiol 42: 97–110, 2013. doi: 10.1093/ije/dys066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Boyd A, Golding J, Macleod J, Lawlor DA, Fraser A, Henderson J, Molloy L, Ness A, Ring S, Davey Smith G. Cohort profile: the ‘Children of the 90s’—the index offspring of the Avon Longitudinal Study of Parents and Children. Int J Epidemiol 42: 111–127, 2013. doi: 10.1093/ije/dys064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Agbaje AO, Barker AR, Tuomainen T-P. Cumulative muscle mass and blood pressure but not fat mass drives arterial stiffness and carotid intima-media thickness progression in the young population and is unrelated to vascular organ damage. Hypertens Res 2022. doi: 10.1038/s41440-022-01065-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Agbaje AO, Lloyd-Jones DM, Magnussen CG, Tuomainen T-P. Cumulative dyslipidemia with arterial stiffness and carotid IMT progression in asymptomatic adolescents: a simulated intervention longitudinal study using temporal inverse allocation model. Atherosclerosis 364: 39–48, 2023. doi: 10.1016/j.atherosclerosis.2022.11.011. [DOI] [PubMed] [Google Scholar]
- 19. Ostchega Y, Porter KS, Hughes J, Dillon CF, Nwankwo T. Resting pulse rate reference data for children, adolescents, and adults: United States, 1999-2008. Natl Health Stat Report 41: 1–16, 2011. [PubMed] [Google Scholar]
- 20. Falaschetti E, Hingorani AD, Jones A, Charakida M, Finer N, Whincup P, Lawlor DA, Davey Smith G, Sattar N, Deanfield JE. Adiposity and cardiovascular risk factors in a large contemporary population of pre-pubertal children. Eur Heart J 31: 3063–3072, 2010. doi: 10.1093/eurheartj/ehq355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Khandaker GM, Zammit S, Lewis G, Jones PB. Association between serum C-reactive protein and DSM-IV generalized anxiety disorder in adolescence: findings from the ALSPAC cohort. Neurobiol Stress 4: 55–61, 2016. doi: 10.1016/j.ynstr.2016.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Troiano RP, Berrigan D, Dodd KW, Mâsse LC, Tilert T, Mcdowell M. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc 40: 181–188, 2008. doi: 10.1249/mss.0b013e31815a51b3. [DOI] [PubMed] [Google Scholar]
- 23. Schielzeth H, Dingemanse NJ, Nakagawa S, Westneat DF, Allegue H, Teplitsky C. Robustness of linear mixed-effects models to violations of distributional assumptions. Methods Ecol Evol 11: 1141–1152, 2020. doi: 10.1111/2041-210X.13434. [DOI] [Google Scholar]
- 24. Browne MW, Cudeck R. Alternative ways of assessing model fit. In: Testing Structural Equation Models, edited by Bollen KA, Long JS.. Newbury Park, CA: Sage, 1993, p. 136–162. [Google Scholar]
- 25. Agbaje AO. Arterial stiffness precedes hypertension and metabolic risks in youth: a review. J Hypertens 40: 1887–1896, 2022. doi: 10.1097/HJH.0000000000003239. [DOI] [PubMed] [Google Scholar]
- 26. Agbaje AO, Barker AR, Tuomainen T-P. Cardiorespiratory fitness, fat mass, and cardiometabolic health with endothelial function, arterial elasticity, and stiffness. Med Sci Sports Exerc 54: 141–152, 2022. doi: 10.1249/MSS.0000000000002757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Golding G, Pembrey P, Jones J, ALSPAC Study Team. ALSPAC—The Avon Longitudinal Study of Parents and Children I. Study methodology. Paediatr Perinat Epidemiol 15: 74–87, 2001. doi: 10.1046/j.1365-3016.2001.00325.x. [DOI] [PubMed] [Google Scholar]
- 28. Ryan SM, Goldberger AL, Pincus SM, Mietus J, Lipsitz LA. Gender- and age-related differences in heart rate dynamics: are women more complex than men? J Am Coll Cardiol 24: 1700–1707, 1994. doi: 10.1016/0735-1097(94)90177-5. [DOI] [PubMed] [Google Scholar]
- 29. Skotsimara G, Antonopoulos AS, Oikonomou E, Siasos G, Ioakeimidis N, Tsalamandris S, Charalambous G, Galiatsatos N, Vlachopoulos C, Tousoulis D. Cardiovascular effects of electronic cigarettes: a systematic review and meta-analysis. Eur J Prev Cardiol 26: 1219–1228, 2019. doi: 10.1177/2047487319832975. [DOI] [PubMed] [Google Scholar]
- 30. Alansare AB, Bates LC, Stoner L, Kline CE, Nagle E, Jennings JR. Associations of sedentary time with heart rate and heart rate variability in adults: a systematic review and meta-analysis of observational studies. Int J Environ Res Public Health 18: 8508, 2021. doi: 10.3390/ijerph18168508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Boutouyrie P, Lacolley P, Girerd X, Beck L, Safar M, Laurent S. Sympathetic activation decreases medium-sized arterial compliance in humans. Am J Physiol Heart Circ Physiol 267: H1368–H1376, 1994. doi: 10.1152/ajpheart.1994.267.4.H1368. [DOI] [PubMed] [Google Scholar]
- 32. Mäki-Petäjä KM, Barrett SML, Evans SV, Cheriyan J, McEniery CM, Wilkinson IB. The role of the autonomic nervous system in the regulation of aortic stiffness. Hypertension 68: 1290–1297, 2016. doi: 10.1161/HYPERTENSIONAHA.116.08035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cartier A, Côté M, Lemieux I, Pérusse L, Tremblay A, Bouchard C, Després J-P. Sex differences in inflammatory markers: what is the contribution of visceral adiposity? Am J Clin Nutr 89: 1307–1314, 2009. doi: 10.3945/ajcn.2008.27030. [DOI] [PubMed] [Google Scholar]
- 34. Kwok S, Canoy D, Ashton WD, Lowe GDO, Wood D, Humphries SE, Charlton-Menys V, Durrington PN. Increased C-reactive protein levels in overweight and obese women taking exogenous hormones: the United Kingdom Women’s Heart Study (UKWHS). Clin Endocrinol (Oxf) 71: 727–732, 2009. doi: 10.1111/j.1365-2265.2009.03580.x. [DOI] [PubMed] [Google Scholar]
- 35. Monteiro R, Teixeira D, Calhau C. Estrogen signaling in metabolic inflammation. Mediators Inflamm 2014: 615917, 2014. doi: 10.1155/2014/615917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Juonala M, Viikari JSA, Rönnemaa T, Taittonen L, Marniemi J, Raitakari OT. Childhood C-reactive protein in predicting CRP and carotid intima-media thickness in adulthood. Arterioscler Thromb Vasc Biol 26: 1883–1888, 2006. doi: 10.1161/01.ATV.0000228818.11968.7a. [DOI] [PubMed] [Google Scholar]
- 37. Buckland G, Northstone K, Emmett PM, Taylor CM. The inflammatory potential of the diet in childhood is associated with cardiometabolic risk in adolescence/young adulthood in the ALSPAC birth cohort. Eur J Nutr 61: 3471–3486, 2022. doi: 10.1007/s00394-022-02860-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cuenca-García M, Ortega FB, Ruiz JR, González-Gross M, Labayen I, Jago R, Martínez-Gómez D, Dallongeville J, Bel-Serrat S, Marcos A, Manios Y, Breidenassel C, Widhalm K, Gottrand F, Ferrari M, Kafatos A, Molnár D, Moreno LA, De Henauw S, Castillo MJ, Sjöström M, HELENA Study Group. Combined influence of healthy diet and active lifestyle on cardiovascular disease risk factors in adolescents. Scand J Med Sci Sports 24: 553–562, 2014. doi: 10.1111/sms.12022. [DOI] [PubMed] [Google Scholar]
- 39. Naqvi TZ, Lee MS. Carotid intima-media thickness and plaque in cardiovascular risk assessment. JACC Cardiovascular Imaging, 2014. doi: 10.1016/j.jcmg.2013.11.014. [DOI] [PubMed] [Google Scholar]
- 40. Wilkinson IB, Mäki-Petäjä KM, Mitchell GF. Uses of arterial stiffness in clinical practice. Arterioscler Thromb Vasc Biol 40: 1063–1067, 2020. doi: 10.1161/ATVBAHA.120.313130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Agbaje AO. Mediating role of body composition and insulin resistance on the association of arterial stiffness with blood pressure among adolescents: the ALSPAC study. Front Cardiovasc Med 9: 939125, 2022. doi: 10.3389/fcvm.2022.939125. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The informed consent obtained from ALSPAC participants does not allow the data to be made freely available through any third-party maintained public repository. However, data used for this submission can be made available on request to the ALSPAC Executive. The ALSPAC data management plan describes in detail the policy regarding data sharing, which is through a system of managed open access. Full instructions for applying for data access can be found here: http://www.bristol.ac.uk/alspac/researchers/access/. The ALSPAC study website contains details of all the data that are available (http://www.bristol.ac.uk/alspac/researchers/our-data/).