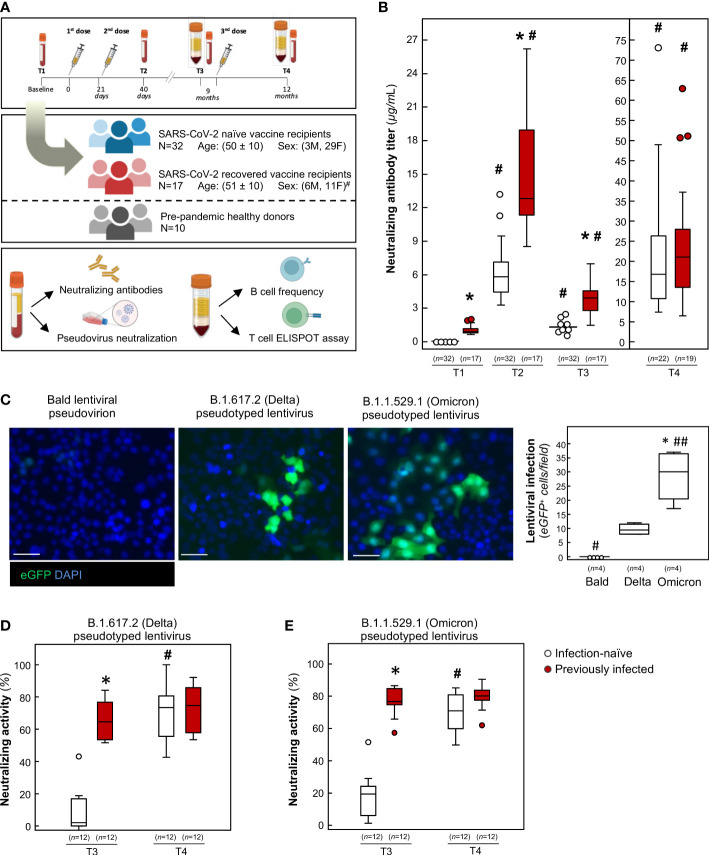
Figure 1.
Humoral response and neutralizing activity of infection-naïve and previously infected vaccine recipients over time. (A) Schematic representation depicting the study design. Drawings were created using BioRender. #p-value=0.049 vs infection-naïve subjects. (B) Evaluation of neutralizing anti-RBD IgG in infection-naïve and previously infected vaccine recipients at baseline (T1), 19 days after primary vaccination (T2), 9 months after primary vaccination (T3), and 3 months after the booster dose (T4). *p-value<0.0001 vs infection-naïve subjects; #p-value<0.0001 vs the respective T1. (C) Representative images and quantification of lentiviral construct infection in Vero E6 cells overexpressing human angiotensin converting enzyme 2 (ACE2). Nuclei are counterstained with Hoechst. Scale bar: 50 μm. *p-value<0.001 vs Bald, #p-value<0.01; ##p-value<0.001 vs Delta. (D, E) Quantification of neutralizing activity of sera against (D) B.1.617.2 (Delta) and (E) B.1.1.529.1 (Omicron) at T3 and T4. *p-value<0.0001 vs infection-naïve subjects; #p-value<0.0001 vs the respective T3. The sample size (n) for each panel is indicated in brackets.