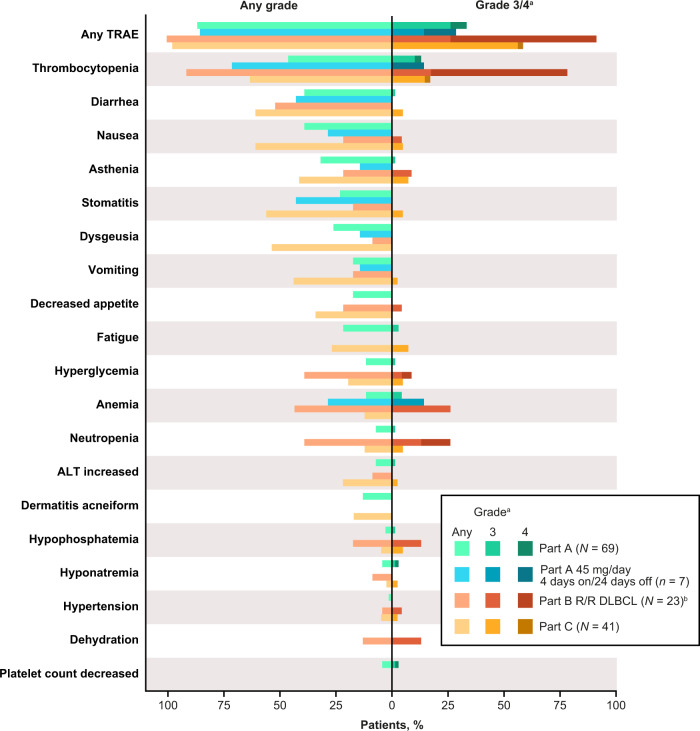
Fig. 2. Treatment-related adverse events reported in ≥10% of patients in the overall study population, or in ≥2 patients at grade ≥3 severity.
Grade 3 essential hypertension, hypokalemia, lipase increased, liver function test abnormal, presyncope, skin hemorrhage, and grade 4 blood creatinine phosphokinase increased, diabetes mellitus, and inappropriate antidiuretic hormone secretion were reported in one patient each in the overall part A population. Grade 3 abdominal infection, blood bilirubin increased, pneumonia and grade 4 blood creatinine increased, febrile neutropenia, leukopenia, and lymphopenia were reported in one patient each in the part B R/R DLBCL population. Grade 3 gamma-glutamyl transferase increased, hyperamylasemia, and syncope were reported in one patient each in the part C population. Source data are provided as a source data file. aNo grade 5 TRAEs were reported during the study. bNineteen patients received trotabresib 45 mg/day 4 days on/24 days off and four patients received trotabresib 30 mg/day 3 days on/11 days off. ALT alanine aminotransferase, DLBCL diffuse large B-cell lymphoma; R/R relapsed/refractory, TRAE treatment-related adverse event.