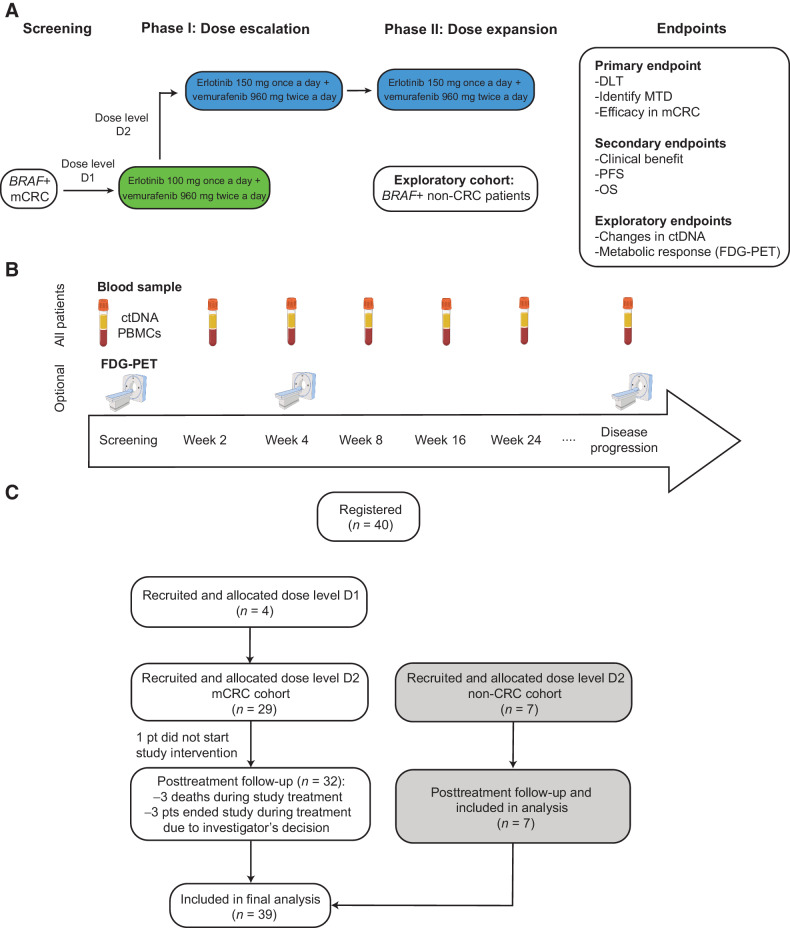
Figure 1.
Design of the EViCT clinical trial. A, Clinical trial design and CONSORT flow diagram. Patients with BRAF V600E positive mCRC were treated with vemurafenib and erlotinib (in escalating doses) in a 3 + 3 phase 1 design. Once the RP2D was determined, an additional 28 patients were enrolled in the colorectal cancer dose expansion cohort and 7 patients in the exploratory non-colorectal cancer dose expansion cohort. B, Study overview and samples for translational research. C, Clinical trial consort diagram with final number of patients recruited and analyzed in dose escalation phase, dose expansion colorectal cancer cohort and dose expansion non-colorectal cancer cohort.