Abstract
An adipose cell (SW872) model was developed to observe cellular necrosis and apoptosis upon Mycobacterium ulcerans infection and treatment with mycobacterial exudate. Apoptosis was likely due to secreted proteins, while necrosis was likely due to mycolactone. Our data suggest that additional factors in M. ulcerans may be involved in Buruli ulcer pathogenesis.
Buruli ulcer (BU) disease, caused by Mycobacterium ulcerans infection, is characterized by chronic necrotizing skin ulcers, with tissue destruction often observed in the adipose tissue and at sites distal to acid-fast bacilli. Based on these observations, the pathogenesis of BU has been hypothesized to be due to one or more secreted mycobacterial toxins. Early studies on the elucidation of a toxin led to the description of a heat-stable and pronase- and lipase-labile factor from the culture filtrate of M. ulcerans (10). Further studies revealed that M. ulcerans filtrates possessed immunosuppressive properties (16). The advent of more sensitive biochemical methods led to the identification and characterization of a unique polyketide in M. ulcerans, named mycolactone, which possesses distinct cytotoxic and immunosuppressive characteristics (4–6, 15).
A perusal of the literature demonstrates that previous studies of the cytotoxicity of M. ulcerans culture filtrate (MUCF) relied upon albumin- or serum-supplemented media. The high protein content of such media impeded the characterization of the secreted proteins of M. ulcerans, including their role, if any, in cytotoxicity. Recent developments in the culture of M. ulcerans in protein-free media have led to the discovery of serodiagnostic proteins (1) and phospholipases (7, 8) in the MUCF and facilitated this study, in which a human adipose cell model of M. ulcerans infection and MUCF cytotoxicity was established.
M. ulcerans infection of human adipose cells.
M. ulcerans strain 94-816 (kindly provided by Francoise Portaels) was grown by serial 10-fold passage to 100 ml in Middlebrook 7H9 oleic acid-albumin-dextrose-catalase broth until the optical density (600-nm λ) was 0.5 to 0.7. Single-cell bacterial suspensions were made by pulse sonication at 4°C using a Branson 450 cell sonicator fitted with a cup horn attachment (Branson Ultrasonics, Danbury, Conn.).
Human adipose cells (SW872) were obtained from the American Type Culture Collection (ATCC HTB 92; Manassas, Va.). Cells were maintained in L-15S (Leibovitz-15 medium with l-glutamine [Gibco/BRL, Grand Island, N.Y.], supplemented with 10% heat-inactivated fetal bovine serum [HyClone Laboratories Inc., Logan, Utah] and sodium bicarbonate [1.5 g/liter; Gibco]) and incubated at 37°C and 5% CO2. Prior to use in experimental assays, the cells were released from the culture flask with 0.25% trypsin (Gibco), washed twice with fresh medium, and seeded onto 6- or 24-well microculture plates as needed. Viable cell counts were confirmed prior to each experiment using trypan exclusion (13).
For electron microscopic (EM) analysis, fresh SW872 cells were trypsinized (0.25.%), washed twice, and adjusted to the desired density with L-15S* (L-15S supplemented with only 1% fetal bovine serum) to 5 × 105 cells/ml. SW872 cells were placed into sterile cryovial tubes and infected with single-cell suspensions of M. ulcerans at a multiplicity of infection of 4:1 (bacterium/cell ratio; confirmed by CFU determination on 7H10 agar plates prior to each infection). Cells and bacteria were incubated with rotation at 32.5 or 37°C for 24 h. The tubes were then centrifuged at 5,000 × g, the medium was removed, and the pellet was washed twice with phosphate-buffered saline (PBS). EM fixative (1% glutaraldehyde in PBS, EM grade) was added, and cells were resuspended by rotation at 4°C for 1 h. The tubes were centrifuged at 5,000 × g, the fixative was removed, the pellet was washed with PBS, and 100 μl of 300 mM cacodylate buffer was added. The samples were embedded as described previously (3), and EM analysis was conducted using a Philips CM-10 transmission electron microscope.
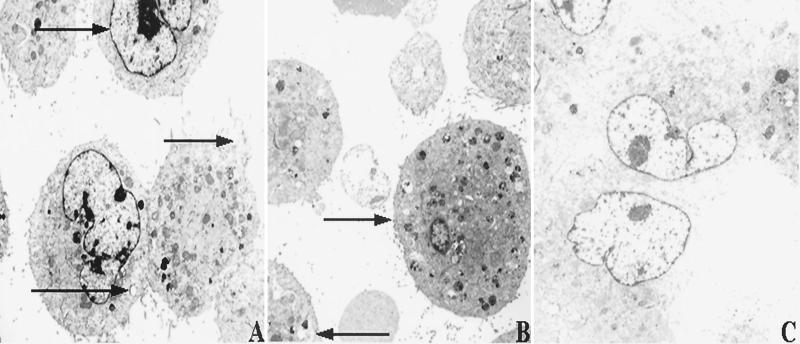
Similar to histological observations of adipose tissues from infected human patients (9, 11), M. ulcerans did not enter SW872 cells upon incubation at either temperature (data not shown). Nonetheless, cytotoxicity was observed within 1 day postinfection at both temperatures. M. ulcerans induced extensive intracellular granule formation, vacuolization, nucleosome condensation, and cell blebbing (Fig. 1A and B). Uninfected cells treated in the identical fashion did not demonstrate similar phenotypes (Fig. 1C). Interestingly, transmission EM analysis of infected SW872 cells demonstrated more apoptotic cells at 32.5°C (Fig. 1A), whereas at 37°C infected cells appeared as both necrotic (or lysed) and apoptotic (Fig. 1B).
FIG. 1.
EM of human adipose cells (SW872) infected with M. ulcerans for 24 h. (A) SW872 cells incubated at 32.5°C undergoing necrosis and apoptosis. Magnification, ×2,950. Arrows denote nucleosome condensation, cell blebbing, and a vacuole, respectively (top to bottom). (B) SW872 cells incubated at 37°C undergoing necrosis and apoptosis. Magnification, ×2,950. Arrows denote a highly granulated and vacuolated cell and a cell with numerous vacuoles, respectively (top to bottom). (C) Uninfected SW872 cells. Magnification, ×2,950.
Visual microscopic analysis of infected SW872 cells was consonant with our EM analyses. The cell monolayers were disrupted within 3 days postinfection at either 32.5 or 37°C, and infected monolayers displayed cytopathic attributes that included granule formation, vacuolization, pseudopodium formation, and cell blebbing (data not shown).
MUCF and mycolactone treatment of adipose cells.
MUCF from protein-free media and mycolactone were prepared and quantitated as described previously (1, 5). In addition, MUCF was tested using the Limulus amebocyte lysate assay (Bio-Whittaker, Walkersville, Md.) for the presence of endotoxin prior to experiments using SW872 cell cultures, and endotoxin units were always less than 0.3 U/4 mg of protein.
Denaturation of MUCF from strain 94-816 was achieved by incubation at 100°C for 30 min (94-816 HD), or by incubation with 200 μg of proteinase K (Sigma Chemical Co., St. Louis, Mo.)/ml for 3 h at 37°C, followed by incubation at 100°C for 30 min (94-816 PKHD).
SW872 cell monolayers, prepared as described above, were treated with 20 μg of MUCF from 94-816, 94-816 HD, or 94-816 PKHD or 0.01, 0.1, 1.0, or 10 μg of purified mycolactone. Cell cultures were incubated at 37°C and 5% CO2 for up to 7 days.
Microscopic analysis of nondenatured MUCF-treated SW872 cell monolayers demonstrated a phenotype similar to that of M. ulcerans-infected cell monolayers within 3 days postexposure, including granule formation, vacuolization, pseudopodium formation, and areas of SW872 cell clearing and cellular debris (data not shown). Heat-denatured MUCF retained this cytotoxic phenotype, while MUCF that was both proteinase K digested and heat denatured appeared to be less cytotoxic (data not shown). Mycolactone-treated cell monolayers displayed only cell rounding and lifting at all concentrations tested, as previously described for this molecule (4, 5; data not shown), suggesting that the MUCF may contain products other than mycolactone with cytotoxic properties.
Quantitative measures of the cytotoxicity induced by MUCF and mycolactone treatment of human adipose cell monolayers were next determined by measuring apoptosis (presence of histone-DNA complexes in cell lysates) and necrosis (release of lactate dehydrogenase [LDH] from permeated and/or leaky cell membranes) from treated SW872 cells.
Apoptosis was measured from MUCF- and mycolactone-treated SW872 cell monolayers at days 1, 3, and 5 posttreatment using the Cell Death Detection Plus enzyme-linked immunosorbent assay (Roche, Indianapolis, Ind.) as described previously for studies of mycobacterial pathogenesis (2). Percent apoptosis was then determined using the following calculation: [(measurement of DNA-histone complex from treated cells − background measurement of untreated cells)/(maximum DNA-histone complex measurement from camptothecin-treated cells − background measurement)] × 100.
For necrosis induction, the release of the cytoplasmic enzyme LDH from treated permeabilized SW872 cells was measured at 1, 3, and 5 days posttreatment using the colorimetric kit from Roche as described previously (2). Percent LDH release was then determined using the following calculation: [(release of LDH from treated cells − background release from untreated cells)/(maximum release of LDH by cell lysis − background release)] × 100.
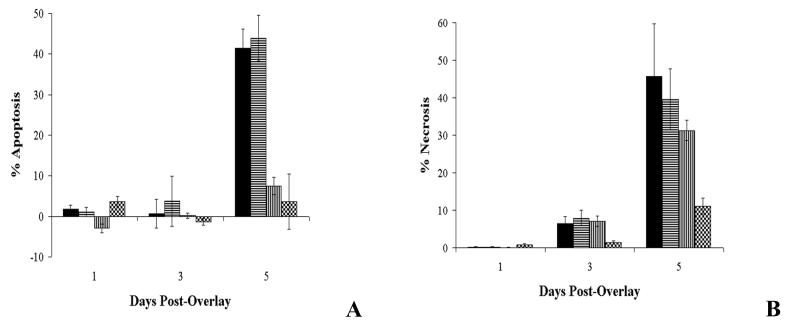
MUCF-treated SW872 cell monolayers demonstrated both apoptosis (Fig. 2A, solid bars) and necrosis (Fig. 2B, solid bars) over the course of exposure. In order to further define the biological nature of the compounds in the MUCF that were responsible for these phenotypes, the MUCF was denatured by heating and enzymatic hydrolysis and the denatured products were tested in this model. Apoptosis induction was significantly reduced when MUCF was first digested with proteinase K and then heat treated prior to addition to SW872 cell monolayers (Fig. 2A, vertically striped bars; Student's t test, P = 0.0011, 94-816 versus 94-816 PKHD, and Student's t test, P = 0.0013, 94-816 HD versus 94-816 PKHD, day 5 postexposure), though no significant difference was found between untreated and heat-denatured MUCF alone (Fig. 2A, horizontally striped bars; Student's t test, P = 0.6802, day 5 postexposure), implicating the action of a heat-stable MUCF protein (or proteins) in the induction of apoptosis of SW872 cells. Conversely, neither heat denaturation alone nor proteinase K treatment and heat denaturation resulted in a significant reduction of necrosis (Fig. 2B, horizontally and vertically striped bars, respectively; Student's t test, P = 0.6494, 94-816 versus 94-816 HD, and Student's t test, P = 0.2657, 94-816 versus 94-816 PKHD, day 5 posttreatment), suggesting that the induction of necrosis was likely to be due to heat- and proteinase-resistant products in the MUCF.
FIG. 2.
Quantitative analysis of necrosis and apoptosis in adipose cell monolayers treated with MUCF or mycolactone. (A) Optical densities of intracellular histone-DNA complexes from the cell lysates of SW872 cell monolayers. (B) Percent LDH release from SW872 cell monolayers. Data shown are the means of triplicate experiments, and error bars represent the standard deviations of these data. The values obtained were normalized by subtraction of background levels of intracellular histone-DNA complexes and of LDH release of uninfected SW872 cell monolayers at each time point. Solid bars, 20 μg of native 94-816 MUCF; horizontally striped bars, 20 μg of heat-denatured 94-816 MUCF; vertically striped bars, 20 μg of proteinase K-treated and heat-denatured 94-816 MUCF; checkered bars, 1.0 μg of mycolactone.
Little apoptosis was induced by purified mycolactone alone (1.0-μg treatment shown; Fig. 2A, checkered bars), and significant differences in apoptosis induction between mycolactone- and MUCF-treated cells were observed (Student's t test, P = 0.0044, day 5 postexposure). In contrast, the induction of necrosis in mycolactone-treated SW872 cell monolayers was not significantly different from the level of necrosis induced by MUCF treatment (Fig. 2B, checkered versus solid bars; Student's t test, P = 0.0439, day 5 postexposure). Cumulatively, these data support the contribution of mycolactone in the induction of necrosis of SW872 cells and the contribution of secreted proteins in the induction of apoptosis of SW872 cell monolayers treated with MUCF.
Biochemical analysis of the MUCF.
Mycolactone induced necrosis in this cell model. As this product has been shown elsewhere to partition to the acetone-soluble lipid (ASL) fraction of cellular lysates and exudates of M. ulcerans (4, 5), it may be in the MUCF and contribute to the necrosis observed with MUCF treatment of SW872 cells.
Thus, the ASL fraction from the MUCF was tested by matrix-assisted laser desorption ionization–time of flight (MALDI-TOF) mass spectrometry for sensitive detection of mycolactone and related products. MALDI-TOF mass spectrometry was conducted using a PerSeptive Biosystems Voyager DE biospectrometry workstation (Applied Biosystems, Inc., Foster City, Calif.) gated for the detection of mycolactone and its derivatives, with an acceleration voltage of 20,000 and a low-mass gate of 300.00 atomic mass units.
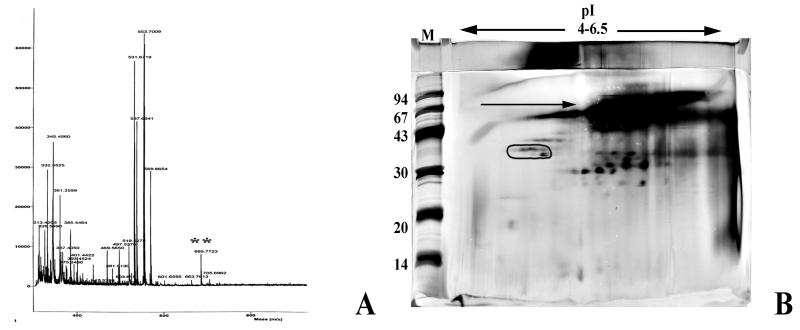
Although a product with the same mass as mycolactone (M + H = 742 [5]) was not found in the MUCF, products with masses identical to those of mycolactone-related structures (M + H = 685 and 786 [P. L. Small, unpublished data]) were observed (Fig. 3A), indicating that mycolactone-related derivatives retained in the MUCF are likely to be responsible for the induction of necrosis in this cell model. This analysis combined with previous thin-layer chromatography of the MUCF demonstrated that these derivatives comprise roughly ≤2% of the MUCF (our unpublished observations).
FIG. 3.
Analysis of the MUCF. (A) Resolution of the ASL fraction of the MUCF by MALDI-TOF mass spectrometry. The double asterisk indicates the presence of a mycolactone-related product. (B) Resolution of the MUCF proteins by two-dimensional gel electrophoresis and staining with silver. Proteins are focused horizontally by pI (range of 4 to 6.5) and vertically by size (range of 100 to 10 kDa). M, molecular weight marker lane, with marker sizes in thousands shown to the left. The circled protein region represents the serodiagnostic 38-kDa protein of the MUCF. The arrow denotes a high-molecular-weight lipoprotein complex in the MUCF.
The MUCF was analyzed by two-dimensional gel electrophoresis using the method of Sonnenberg and Belisle for resolution of mycobacterial proteins (19) and demonstrated numerous proteins, including the serodiagnostic 38-kDa protein (1) (Fig. 3B). Interestingly, a high-molecular-weight lipoprotein complex was abundant in the MUCF (Fig. 3B). The attributes of this complex are similar to those described for other factors previously suggested for the toxin(s) from the culture filtrate of M. ulcerans (10).
Our studies demonstrated the multifactorial cytotoxicity observed in M. ulcerans-infected SW872 cell monolayers (Fig. 1 and data not shown). Interestingly, both apoptosis and necrosis were observed in this cell model, providing a use for this model in the inclusive characterization of the cytotoxic products from M. ulcerans. Further, these phenotypes were readily observed upon infection at 37°C, even though M. ulcerans grows poorly above 35°C (20), suggesting that M. ulcerans cytotoxicity is independent of replication, in contrast to other models of mycobacterial pathogenesis (3, 17).
Treatment of SW872 cell monolayers with MUCF corroborated these analyses. The MUCF was cytotoxic to SW872 cell monolayers and induced both necrosis and apoptosis (Fig. 2). Further, the factors present in the MUCF responsible for necrosis were both heat and proteinase K stable and thus likely to be comprised of mycolactone and related products (Fig. 2B). These analyses also demonstrated that the MUCF-derived factors driving apoptosis were proteinase K sensitive, substantiating a role for the secreted proteins of M. ulcerans in the cytotoxicity of SW872 cells (Fig. 2A).
Mass spectrometry analysis revealed trace quantities of mycolactone-related products in the MUCF (Fig. 3A), and purified mycolactone induced necrosis, supporting its role in the cytotoxicity of human adipose cells (Fig. 2B). In contrast, only 3% of the mycolactone-treated cell monolayers were apoptotic (Fig. 2A), suggesting that apoptosis is a secondary effect of necrosis induction by mycolactone in this cell model. This finding is consistent with other reports for mycolactone (6) and other cytotoxic bacterial factors (12).
Preliminary analyses of the proteins present in the MUCF by two-dimensional gel electrophoresis did not readily identify one protein responsible for apoptosis, though they suggested a high-molecular-weight lipoprotein complex that may contribute to SW872 cell cytotoxicity (Fig. 3B and our unpublished observations). This complex demonstrates characteristics consistent with products previously suggested as toxigenic components of the MUCF (10, 18).
Others have identified cytotoxic properties of the MUCFs in protein-free media (14) and have defined phospholipase activities (7, 8) and cytolysins (A. Mve-Obiang, A. Gomez, J. Bujnicki, R. Kotlowski, L. Rychlewski, J. Remacle, F. Portaels, and P. A. Fonteyne, unpublished data) from the M. ulcerans exudate that may additionally contribute to the cytotoxicity seen in this cell model. Future studies will focus on the utility of this cell model in the delineation of the factors from the MUCF directing SW872 cellular apoptosis and their role in the pathogenesis of BU.
Acknowledgments
This study was supported in part by a grant from the Department of Health and Human Services, U50/CC416560-01.
We acknowledge Kristine Birkness at the Centers for Disease Control and Prevention for her contribution to the preliminary tissue culture studies and the Emory University Microscopy Core for processing the samples for EM analysis.
REFERENCES
- 1.Dobos K M, Spotts E A, Marston B J, Horsburgh C R, Jr, King C H. Serologic response to culture filtrate antigens of Mycobacterium ulcerans during Buruli ulcer disease. Emerg Infect Dis. 2000;6:158–164. doi: 10.3201/eid0602.000208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dobos K M, Spotts E A, Quinn F D, King C H. Necrosis of lung epithelial cells during infection with Mycobacterium tuberculosis is preceded by cell permeation. Infect Immun. 2000;68:6300–6310. doi: 10.1128/iai.68.11.6300-6310.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fischer L J, Quinn F D, White E H, King C H. Intracellular growth and cytotoxicity of Mycobacterium haemophilum in a human epithelial cell line (Hec-1-B) Infect Immun. 1996;64:269–276. doi: 10.1128/iai.64.1.269-276.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.George K M, Barker L P, Welty D M, Small P L. Partial purification and characterization of biological effects of a lipid toxin produced by Mycobacterium ulcerans. Infect Immun. 1998;66:587–593. doi: 10.1128/iai.66.2.587-593.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.George K M, Chatterjee D, Gunawardana G, Welty D, Hayman J, Lee R, Small P L. Mycolactone: a polyketide toxin from Mycobacterium ulcerans required for virulence. Science. 1999;283:854–857. doi: 10.1126/science.283.5403.854. [DOI] [PubMed] [Google Scholar]
- 6.George K M, Pascopella L, Welty D M, Small P L. A Mycobacterium ulcerans toxin, mycolactone, causes apoptosis in guinea pig ulcers and tissue culture cells. Infect Immun. 2000;68:877–883. doi: 10.1128/iai.68.2.877-883.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gomez A, Mve-Obiang A, Vray B, Remacle J, Chemlal K, Meyers W M, Portaels F, Fonteyne P A. Biochemical and genetic evidence for phospholipase C activity in Mycobacterium ulcerans. Infect Immun. 2000;68:2995–2997. doi: 10.1128/iai.68.5.2995-2997.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gomez A, Mve-Obiang A, Vray B, Rudnicka W, Shamputa I C, Portaels F, Meyers W M, Fonteyne P A, Realini L. Detection of phospholipase C in nontuberculous mycobacteria and its possible role in hemolytic activity. J Clin Microbiol. 2001;39:1396–1401. doi: 10.1128/JCM.39.4.1396-1401.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hayman J. Out of Africa: observations on the histopathology of Mycobacterium ulcerans infection. J Clin Pathol. 1993;46:5–9. doi: 10.1136/jcp.46.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hockmeyer W T, Krieg R E, Reich M, Johnson R D. Further characterization of Mycobacterium ulcerans toxin. Infect Immun. 1978;21:124–128. doi: 10.1128/iai.21.1.124-128.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Horsburgh C R, Jr, Meyers W M. Buruli ulcer. In: Horsburgh C R Jr, Nelson A M, editors. Pathology of emerging infections. Washington, D.C.: ASM Press; 1997. pp. 119–134. [Google Scholar]
- 12.Lai X H, Arencibia I, Johansson A, Wai S N, Oscarsson J, Kalfas S, Sundqvist K G, Mizunoe Y, Sjostedt A, Uhlin B E. Cytocidal and apoptotic effects of the ClyA protein from Escherichia coli on primary and cultured monocytes and macrophages. Infect Immun. 2000;68:4363–4367. doi: 10.1128/iai.68.7.4363-4367.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mehta P K, King C H, White E H, Murtagh J J, Jr, Quinn F D. Comparison of in vitro models for the study of Mycobacterium tuberculosis invasion and intracellular replication. Infect Immun. 1996;64:2673–2679. doi: 10.1128/iai.64.7.2673-2679.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mve-Obiang A, Remacle J, Palomino J C, Houbion A, Portaels F. Growth and cytotoxic activity by Mycobacterium ulcerans in protein-free media. FEMS Microbiol Lett. 1999;181:153–157. doi: 10.1111/j.1574-6968.1999.tb08838.x. [DOI] [PubMed] [Google Scholar]
- 15.Pahlevan A A, Wright D J, Andrews C, George K M, Small P L, Foxwell B M. The inhibitory action of Mycobacterium ulcerans soluble factor on monocyte/T cell cytokine production and NF-kappa B function. J Immunol. 1999;163:3928–3935. [PubMed] [Google Scholar]
- 16.Pimsler M, Sponsler T A, Meyers W M. Immunosuppressive properties of the soluble toxin from Mycobacterium ulcerans. J Infect Dis. 1988;157:577–580. doi: 10.1093/infdis/157.3.577. [DOI] [PubMed] [Google Scholar]
- 17.Ramakrishnan L, Falkow S. Mycobacterium marinum persists in cultured mammalian cells in a temperature-restricted fashion. Infect Immun. 1994;62:3222–3229. doi: 10.1128/iai.62.8.3222-3229.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Read J K, Heggie C M, Meyers W M, Connor D H. Cytotoxic activity of Mycobacterium ulcerans. Infect Immun. 1974;9:1114–1122. doi: 10.1128/iai.9.6.1114-1122.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sonnenberg M G, Belisle J T. Definition of Mycobacterium tuberculosis culture filtrate proteins by two-dimensional polyacrylamide gel electrophoresis, N-terminal amino acid sequencing, and electrospray mass spectrometry. Infect Immun. 1997;65:4515–4524. doi: 10.1128/iai.65.11.4515-4524.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsang A Y, Farber E R. The primary isolation of Mycobacterium ulcerans. Am J Clin Pathol. 1973;59:688–692. doi: 10.1093/ajcp/59.5.688. [DOI] [PubMed] [Google Scholar]