Abstract
Although advances in insulin therapy and delivery have been made, global evidence indicates sub-optimal glycemic management in people on insulin therapy with either type 1 diabetes (T1D) or type 2 diabetes (T2D). In this review, we discuss connected insulin pens that include tracking insulin pens (TIPs) and smart insulin pens (SIPs) and caps, as approaches to improving mean glucose or time in range while minimizing exposure to hypoglycemia or time below range (TBR) in people with diabetes (PwD) on multiple daily injection (MDI) therapy. We discuss various factors offered by SIPs that can facilitate precision insulin management, that is, delivering the right dose at the right time. These factors include the automatic recording of insulin dose size and delivery time; differentiating prime from therapy doses; active insulin tracking; dose calculators that provide individualized dosing recommendations; alerts for missed doses (ie, rapid-acting or long-acting insulin), insulin temperature, and insulin age monitoring; and integrated data reports for the clinical care team. A data-driven approach to care is critical to precision insulin management and includes helping PwD make informed choices regarding their preferred method of insulin delivery and ensuring insulin delivery technology tools are configured for their personal therapy plan. The data-driven approach involves developing a plan for ongoing collaborative use of the resulting data with their care team that may include adjusting insulin regimen and optimizing the care plan on a timely basis. We conclude with a list of practice protocols that are needed to support data-driven precision insulin management. This review includes a summary of research including various stages of connected insulin pens and caps.
Keywords: connected insulin pens, data-driven, digital health, multiple daily insulin therapy, numeracy, precision insulin management, smart insulin pen, tracking insulin pen, therapeutic inertia, value-based care
Introduction
Out of the nearly 8 million individuals on insulin therapy in the United States, more than 70% of people with type 1 diabetes (T1D) and more than 99% of people with type 2 diabetes (T2D) who require mealtime and correction insulin rely on injection therapy. 1 Connected insulin pens include tracking insulin pens (TIPs) such as NovoPen 6 and NovoPen Echo by Novo Nordisk (Bagsværd, Denmark) and smart insulin pens (SIPs), like the InPen system by Medtronic Diabetes (Northridge, California, USA) and smart insulin caps such as the BigFoot Unity diabetes management system by BigFoot Biomedical (Milpitas, California, USA) compatible with commercially available disposable insulin pens from major insulin manufacturers. 2
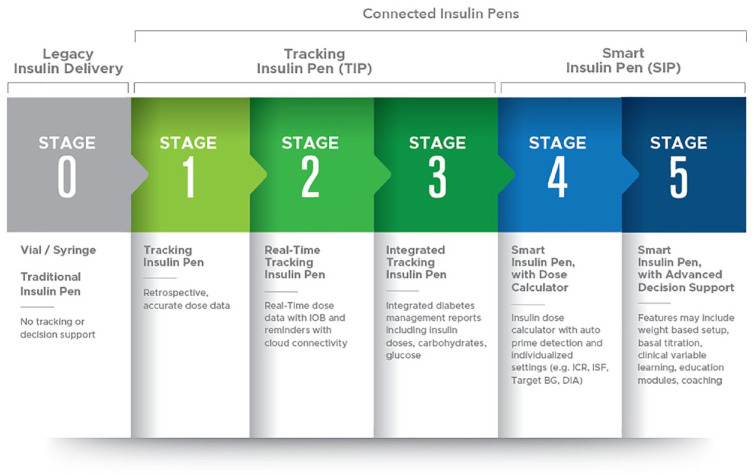
The various stages of connected insulin pen development are shown and described in Figure 1. These devices provide more visibility into insulin dosing behavior and, in the case of SIPs, intelligent dosing support with a simple, durable insulin pen paired to a diabetes management app 3 that addresses challenges in optimizing insulin injection therapy. These challenges include accurately delivering the intended dose; helping to avoid missed, mis-timed, and mismatched insulin doses (ie, delivering the right dose at the right time); as well as providing dosing data integrated with related diabetes data to help inform therapy decisions and overcome therapeutic inertia.
Figure 1.
Roadmap to smart insulin pens. Abbreviations: ICR, insulin-to-carbohydrate ratio; ISF, insulin sensitivity factor; BG, Blood Glucose; DIA, Duration of Insulin Action.
Accurate Dose Delivery (Precision Dosing)
Insulin pens have consistently outperformed syringes as insulin delivery devices due to their greater accuracy and dosing precision, ease of use, and patient preference. 4 These advantages make them better suited to administer insulin, especially in insulin-sensitive pediatric or geriatric individuals on lower doses of rapid-acting insulin. 4 The ISO11608-1 5 international performance standard for pen injectors states that devices must demonstrate accuracy at their specified dosing increment after being subjected to temperature and humidity extremes, vibration, free-fall drop, and dust and moisture exposure. A 2020 review by Aanstoot et al 6 focused on precision dosing of rapid-acting insulin that accurately delivers the intended dose using traditional insulin pens, and emphasized the importance of insulin pens with half-unit dosing capability. For individuals with small dose requirements, this allows more flexible dosing for mealtimes, physical activity, managing sick days, and correction dose delivery in smaller dose increments and can be particularly important for pediatric populations. The InPen SIP, the first FDA-cleared SIP, 7 and the NovoPen Echo are among the five available insulin pens in the United States providing half-unit dosing increments. Currently, the BigFoot Unity caps are compatible with rapid-acting insulin pens dispensing in one-unit increments.
Priming before each insulin dose and ensuring insulin integrity are also important to precision dosing. Priming removes the air from the needle and cartridge that may collect during normal use, helping to ensure the full therapy dose is delivered. An SIP automatically records insulin doses and is able to distinguish prime from therapy doses. This is necessary to accurately track active insulin on board from previously administered doses, and is required for safe and accurate dose calculator functionality. In addition, some connected insulin pens have temperature and age sensors and will alert the user if the insulin in the pen has been exposed to unsafe temperatures or is approaching its expiration date.8,9
Connected insulin pens cannot, however, take the place of appropriate injection technique. Therefore, it is critical that diabetes care teams educate patients on correct insulin injection technique and the rotation of injection sites, as well as assuring insulin integrity. It is reported that approximately 65% of individuals using insulin have lipohypertrophy and that there should be routine (at least annual) physical examinations of insulin injection sites, in addition to an ongoing best practice of recommended pen use and skin care. 10
Delivering the Right Dose at the Right Time
Missed and Mistimed Insulin Doses
In a systematic review of studies reporting on missed and mistimed insulin doses in people with diabetes (PwD), 20% to 45% reported mistiming of their insulin doses 11 citing disrupted daily routines, social situations, and hypoglycemia avoidance as reasons. Connected insulin pens are creating opportunities for health care professionals to have a more complete picture of the pharmaco-adherence of insulin dosing, allowing for more personalized prescribing practices. 12
Research from participants on multiple daily injection (MDI) therapy using continuous glucose monitoring (CGM) devices reports that one in four meals are associated with either a late or missed insulin bolus 13 and occur in individuals with either T1D or T2D across the age spectrum. Doses may be forgotten or intentionally omitted for various reasons, including cost, inconvenience, schedule disruption, or eating disorder. In fact, insulin omission was reported to occur in 100% of participants in a study with a TIP. 14 Omitting two meal-related doses per week has been shown to be associated with a 0.4% increase in HbA1c levels. 15
In a prospective, observational study of 50 participants with T1D on MDI therapy using both a TIP and CGM, 37% of total boluses resulted in persistent hyperglycemia three hours postprandially (glucose ≥ 180 mg/dL), while 10% resulted in clinically significant hypoglycemia (glucose < 55 mg/dL), three hours postprandially. 16 Late boluses (a rise in glucose of ≥ 50 mg/dL before a bolus) occurred twice per patient per week, while missed boluses (indicated by a rise in glucose of ≥50 mg/dL without a prior bolus within two hours) occurred 17 times per patient per week and were associated with higher A1C (R = 0.1, P = .02).
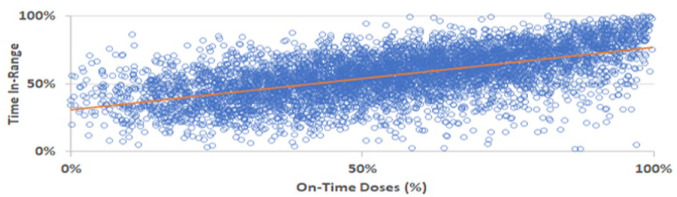
In a retrospective analysis, SIP doses, CGM data, and a rate-of-change detection methodology were used to demonstrate missed, late, and on-time meal-related insulin dosing behaviors and their impact on glycemia in pediatric and adult patients with both T1D and T2D on MDI therapy. 17 Data analyses indicated that 56% of meals were On-Time, 12% were Late, and 32% were Missed. The frequency of On-Time dosing was significantly correlated with user time in range (TIR; R = 0.59, P < .001; Figure 2). Importantly, SIPs can provide missed dose alerts when users neglect to dose during a pre-determined meal-dose time frame, thus helping PwD avoid forgotten doses. If the user is unsure whether they dosed or not, they can check their SIP app to determine when they last dosed, how much was dosed, and the amount of active insulin from previous doses to help avoid over-dosing or missing doses.
Figure 2.
Frequency of on-time dosing correlated with time in range.
Mismatched Mealtime Doses and the Need for Correction Doses
Limited diabetes-related numeracy skills have been reported to potentially affect glycemic management.18,19 Zaugg et al 20 estimated that two out of three adults in the United States. are unable to perform basic math due to limited numeracy skills and that, 25% of PwD could not determine if glucose values were within an identified target range; 56% could not correctly count the carbohydrates in labeled food; and 59% could not calculate an insulin dose when provided a glucose measurement and specific carbohydrate in grams. 20 Overlapping rapid-acting insulin doses, known as insulin stacking, adds another layer of risk (ie, hypoglycemia) and is estimated to occur in 60% of insulin bolus doses. 21
SIPs support patients on MDI therapy with a dose calculator (clinical decision support) providing dose recommendations for food and correction doses based on individualized insulin therapy settings, while accounting for active insulin (insulin-on-board) from previous doses. 8 The SIP dose calculator offers support for those on simpler but less precise fixed mealtime dose regimens, as well as for those who adjust their doses based on the relative size of their meal, or those who count carbohydrates and use an insulin-to-carbohydrate ratio (ICR) for dose calculations. 22 The SIP can vary the insulin therapy settings to decimal-point precision over four time periods, thus accounting for variability in insulin sensitivity, eating habits, and physical activity during the day.
Using SIPs to calculate and deliver more frequent correction doses to safely improve glycemic management is changing the treatment paradigm for MDI therapy. A retrospective analysis of real-world data of 1721 individuals (66% T1D; all ages) using the SIP, with CGM demonstrated a significant difference in TIR across groups, where the most significant increase in TIR was observed when ≥6 injections per day were administered compared with groups administering one to four injections per day. 23 The average time below range (TBR) remained no more than 3% across all groups. In a subsequent analysis of 2816 InPen users, 24 data revealed a significant increase in the frequency of correction-only doses by users who dosed more frequently per day (43% for users with ≥6 doses/day) compared with those who dosed less frequently (28%-33% for users with 1 to ≤4 doses/day), while maintaining TBR (2.8%) within clinical consensus guidelines. 25
Providing adequate training and ongoing support is critical to the adoption of any diabetes technology. 26 The diabetes care team can encourage PwD using SIP to regularly use the dose calculator, to follow the dose recommendations and to set a reminder for taking mealtime doses and checking their glucose two hours after a meal to determine if a correction dose is needed. Providing PwD with individualized calculated insulin therapy settings can help them follow the dose calculator recommendations as advised and have confidence in the recommendations. 27
Data-Driven Precision Insulin Management
The Association of Diabetes Care & Education Specialists (formerly, the American Association of Diabetes Educators) adopted the Identify-Configure-Collaborate framework as a standardized approach for integrating any diabetes technology into clinical practice to help improve technology access, adoption, and, ultimately, positive outcomes through data-driven care.28,29 A data-driven approach to care is critical to precision insulin management and includes three hallmarks guided by shared decision making: (1) Identify: helping PwD make informed choices regarding their preferred method of insulin delivery; (2) Configure: assuring insulin delivery technology tools are configured for the personal therapy plan and circumstances of PwD; and (3) Collaborate: developing a plan for ongoing collaborative use of the resulting data by PwD and their care team, to adjust the insulin regimen, fine-tune insulin therapy settings, and optimize the care plan on a timely basis.
Identify
It is important that, as a standard of care, care teams help every person on insulin therapy make an informed decision about their preferred method of insulin delivery and consider convenience, cost, complexity, and the availability of intelligent technology features that can support precision insulin management: whether for SIPs or automated insulin delivery devices. The American Diabetes Association (ADA) Standards of Medical Care in Diabetes note, there is no “one-size-fits-all” approach to technology use in diabetes care, and individuals should be supported in technology options that best match their circumstances, desires, and needs. 26 The standards recommend that connected insulin pen therapies can be helpful for diabetes management and that US Food and Drug Administration–approved insulin dose calculators/decision support systems may be helpful for titrating insulin doses. 26
The advent of SIPs has opened the digital door to a much larger population of PwD, providing daily insulin management decision support and integrated diabetes data for the first time for care teams. 30 Smart insulin pens are part of a growing ecosystem of connected devices and apps expanding connectivity to better support PwD and reduce the burden of care and also enable remote monitoring by care partners and/or care teams when desired. These connected devices include eating behavior detection apps such as Klue (Klue, Inc., Vancouver, British Columbia, Canada) and fitness trackers and sleep monitors among others. 31 The KLUE application detects eating-hand gestures using motion sensors that an algorithm converts to carbohydrate amounts that could be relayed to a SIP dose calculator or automated insulin delivery (AID) system thus potentially alleviating the burden of carbohydrate counting. This technology has been effective in feasibility studies with AID system but has not yet been applied to insulin injection therapy. 32
Configure
When prescribing a device, the ADA Standards of Medical Care in Diabetes recommend that health care teams ensure that people with diabetes and their caregivers receive initial and ongoing education and training, including the ability to use and share data to adjust therapy, noting that provider input and education can be helpful for setting the initial dosing calculations with ongoing follow-up for adjustments as needed. There may be a learning curve for many who are using new technologies. 26 However, with ongoing help in configuring the technology (Table 1), so that it works with the individual’s therapy plan, PwD can ultimately benefit as more active participants in their care.
Table 1.
SIP Onboarding Configuration Steps.
| SIP onboarding configuration steps |
|---|
| 1. Calculate and provide individualized insulin therapy settings and assist with programming the app as needed. |
| 2. Assure that the meal schedule in the app is adjusted to the person’s daily routine; turn on desired missed dose alerts and glucose check reminders. Connect to available Bluetooth-enabled glucose monitoring devices in use. |
| 3. Agree on a plan for sharing the data with the care team. |
| 4. Assure the patient has current prescriptions for basal insulin, rapid-acting insulin cartridges, pen needles, and connected glucose monitoring supplies. Address any cost concerns. Assure a backup plan is available for insulin injections. |
| 5. Teach or check insulin injection technique, injection site rotation, importance of priming, and proper insulin storage. |
| 6. Set clear expectations regarding use of SIP as part of
daily self-management: • Unsure if took dose: Advise to check the app home screen for the last dose amount and time, last glucose, and any active insulin. • Forgets doses: Set missed dose alerts for usual mealtimes. • Unsure how much to dose for meals and corrections: Advise to check glucose and use the bolus calculator when dosing. • While fine-tuning settings and learning carbohydrate counting, ask the patient to check their glucose two hours after their mealtime doses to determine if a correction dose is needed. • Keeping records of diabetes data: Encourage patients to log additional insulin doses such as long-acting insulin and short-acting doses taken without SIP in the Logbook. Suggest patients review their Logbook as needed for daily history and adjust reminders for doses and glucose checks as needed. • Upcoming care team touchpoint: Ask patients to share data reports and engage with the care team at and between health care visits per plan. • The care plan has been adjusted: Update Insulin Therapy Settings as care plan evolves. |
Abbreviation: SIP, smart insulin pen.
Collaborate
Critical to adoption of technology and ongoing engagement is the collaborative use of the resulting data with the care team to assess and address barriers to following the agreed-on care plan and to incrementally modify the care plan to optimize therapy on a timely basis. 27 Connected devices, such as SIPs with CGM, provide data for remote patient monitoring and more timely touchpoints as needed with the care team. This opens the door to continuous data-driven care, fostering shared decision making and reducing therapeutic inertia. 27 Establishing clear roles and responsibilities for data workflow in clinical practice is essential to determining how and when data reports will be received, determining who will review the data reports, and assuring the right team members respond with appropriate therapy recommendations in a continuous feedback loop. Integrated data reports from SIPs are reported to improve the quality of the visit 33 and lead to more collaborative and less interrogative patient-provider conversations. A reference guide for building a data-driven practice model for MDI therapy using SIPs was published in 2020 and includes a method for systematic review of SIP data and a person-centered counseling strategy. 34
Practice Protocols Needed for Precision Insulin Management
Data from connected insulin pens have revealed significant gaps and opportunities in MDI therapy care. For example, in a retrospective real-world data analysis, SIP users were shown to have fasting glucose levels outside recommended standards and significant differences between bedtime and fasting glucose levels, revealing a need for basal dose optimization. 35 These gaps uncover the need for developing various practice protocols for data-driven precision insulin management including
Teaching and verifying appropriate insulin injection technique, site rotation, timing of insulin dose relative to eating, priming, safe insulin storage and rotation of supplies;
Annual physical palpation of insulin injection sites and checks on insulin injection technique (or more often if unexplained hyperglycemia);
Optimizing the long-acting (basal) insulin dose and identifying when to add prandial insulin in T2D;
Establishing rapid-acting insulin therapy settings, including target glucose, duration of insulin action or active insulin time, ICR or meal doses, and insulin sensitivity factor (ISF) or correction factor to match individual needs;
SIP data review including refining the insulin therapy settings and overall care plan on an ongoing basis, with procedures for virtual care and remote monitoring;
Prescribing and training on the use of CGM for patients on insulin therapy as per current standards of care. If using blood glucose monitoring, provide optimal blood glucose monitoring schedules to support the prescribed insulin regimen.
Much of this work will be automated as Stage 5 SIP technology advances (Smart MDI Therapy, a SIP integrated with real-time CGM data) and will increasingly reduce the burden of care for both the patient and the clinical team potentially with cost savings. A health economic analysis with a connected insulin pen demonstrated improved outcomes with lower cost when integrating the data into the clinical care process. 36 Smart MDI therapy could support weight-based initiation and ongoing adjustment of insulin therapy settings such as ISF, dose titration when needed, and data-driven automated education and prompts to support the patient in daily self-management and clinical decision making.
Conclusion
Data-driven precision insulin management is possible with the advent of SIPs that support PwD on MDI therapy. Health care teams are encouraged to embrace these capabilities and to develop person-centric practice protocols to address the unmet needs of their diabetes population on insulin injection therapy. This may, in turn, improve diabetes technology access, adoption, and, ultimately, outcomes. Further research including long-term studies and randomized controlled trials is needed to demonstrate the efficacy of connected insulin pens including TIPs and SIPs and smart caps particularly when integrated with real-time CGM data.
Footnotes
Abbreviations: ADA, American Diabetes Association; CGM, continuous glucose monitoring; FDA, Food and Drug Administration; MDI, multiple daily insulin therapy; PGHD, patient-generated health data; PwD, people with diabetes; SIP, smart insulin pen; TIP, tracking insulin pen.
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Both authors are employees and stockholders in Medtronic Diabetes.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Janice MacLeod  https://orcid.org/0000-0001-8689-4938
https://orcid.org/0000-0001-8689-4938
Robert A. Vigersky  https://orcid.org/0000-0002-3546-3385
https://orcid.org/0000-0002-3546-3385
References
- 1. Sikes KA, Weyman K. Diabetes and the use of insulin pumps. Endocrinology Advisor. https://www.endocrinologyadvisor.com/home/decision-support-in-medicine/endocrinology-metabolism/diabetes-and-the-use-of-insulin-pumps/. Published January 2019. Accessed October 15, 2022.
- 2. Steenkamp D, Eby EL, Gulati N, Liao B. Adherence and persistence to insulin therapy in people with diabetes: impact of connected insulin pen delivery ecosystem. J Diabetes Sci Technol. 2022;16(4):995-1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kerr D, Warshaw H, Choi NY. Smart insulin pens will address critical unmet needs for people with diabetes using insulin. Endocr Today. 2019;17(5):21-22. [Google Scholar]
- 4. Luijf YM, DeVries JH. Dosing accuracy of insulin pens versus conventional syringes and vials. Diabetes Technol Ther. 2010;12(suppl 1):S73-S77. [DOI] [PubMed] [Google Scholar]
- 5. International Organization for Standardization. Needle-based injection systems for medical use—requirements and test methods—part 1: needle-based injection systems (ISO 11608-1:2022). https://www.iso.org/standard/70733.html. Published January 2022. Accessed October 15, 2022.
- 6. Aanstoot HJ, Rodriguez H, Weinzimer S, Vint N, Koeneman L. Precision dosing of rapid-acting insulin matters. Diabetes Technol Ther. 2020;22(5):346-351. [DOI] [PubMed] [Google Scholar]
- 7. Food and Drug Administration. Companion medical InPen system (K160629) clearance letter. https://www.accessdata.fda.gov/cdrh_docs/pdf16/K160629.pdf. Published July 26, 2016. Accessed October 15, 2022.
- 8. Bailey TS, Stone JY. A novel pen-based Bluetooth-enabled insulin delivery system with insulin dose tracking and advice. Expert Opin Drug Deliv. 2017;14(5):697-703. [DOI] [PubMed] [Google Scholar]
- 9. Sangave NA, Aungst TD, Patel DK. Smart connected insulin pens, caps and attachments: a review of the future of diabetes technology. Diabetes Spectr. 2019;32(4):378-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Frid AH, Kreugel G, Grassi G, et al. New insulin delivery recommendations. Mayo Clin Proc. 2016;91(9):1231-1255. [DOI] [PubMed] [Google Scholar]
- 11. Robinson S, Newson RS, Liao B, Kennedy-Martin T, Battelino T. Missed and mistimed insulin doses in people with diabetes: a systematic literature review. Diabetes Technol Ther. 2021;23(12):844-856. [DOI] [PubMed] [Google Scholar]
- 12. Klonoff DC, Zhang JY, Shang T, et al. Pharmacoadherence: an opportunity digital health to inform the third dimension of phamacotherapy for diabetes. 2021;15(1):177-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Norlander LM, Anderson S, Levy CJ, et al. Late and missed meal boluses with multiple daily insulin injections. Diabetes. 2018;67(suppl 1):A259. [Google Scholar]
- 14. Munshi MN, Slyne C, Greenberg JM, et al. Nonadherence to insulin therapy detected by Bluetooth-enabled pen cap is associated with poor glycemic control. Diabetes Care. 2019;42(6):1129-1131. [DOI] [PubMed] [Google Scholar]
- 15. Randlov J, Poulsen JU. How much do forgotten insulin injections matter to hemoglobin A1c in people with diabetes? A simulation study. J Diabetes Sci Technol. 2008;2(2):229-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Toschi E, Slyne C, Greenberg JM, et al. Examining the relationship between pre- and postprandial glucose levels and insulin bolus timing using Bluetooth-enabled insulin pen cap technology and continuous glucose monitoring. Diabetes Technol Ther. 2020;22(1):19-24. [DOI] [PubMed] [Google Scholar]
- 17. Smith M, Thanasekaran S, Im G, et al. Impact of missed, late and on-time meal-related dosing behaviors during InPen smart insulin pen use. Poster (IP22) presentation at the Association of Diabetes Care & Education Specialists Virtual Conference; August 2021; Baltimore, MD. [Google Scholar]
- 18. Marden S, Thomas PW, Sheppard ZA, Knott J, Lueddeke J, Kerr D. Poor numeracy skills are associated with glycaemic control in type 1 diabetes. Diabet Med. 2012;29(5):662-669. [DOI] [PubMed] [Google Scholar]
- 19. Cavanaugh K, Huizinga MM, Wallston KA, et al. Association of numeracy and diabetes control. Ann Intern Med. 2008;148(10):737-746. [DOI] [PubMed] [Google Scholar]
- 20. Zaugg SD, Dogbey G, Collins K, et al. Diabetes numeracy and blood glucose control: association with type of diabetes and source of care. Clin Diabetes. 2014;32(4):152-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schmidt S, Norgaard K. Bolus calculators. J Diabetes Sci Technol. 2014;8(5):1035-1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Companion Medical. FDA clears InPen diabetes management system for fixed dosing and meal estimation. Cision™ PR Newswire. February 27, 2020. https://www.prnewswire.com/news-releases/fda-clears-inpen-diabetes-management-system-for-fixed-dosing-and-meal-estimation-301013128.html. Accessed October 15, 2022.
- 23. Smith M, Thanasekaran S, Gaetano A, et al. Increased numbers of daily injections with smart insulin pens improves glycemia. Poster presentation at the Association of Diabetes Care & Education Specialists Virtual Conference; August 2020; Baltimore, MD. [Google Scholar]
- 24. Smith M, Thanasekaran S, Im G, et al. Smart insulin pens allows correction doses as needed without compromising time below range. J Diabetes Sci Technol. 2020;15(2):A69. [Google Scholar]
- 25. Battelino T, Danne T, Bergenstal RM, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the International Consensus on Time in Range. Diabetes Care. 2019;42(8):1593-1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. American Diabetes Association Professional Practice Committee. Diabetes technology: standards of medical care in diabetes—2022. Diabetes Care. 2022;45(suppl 1):S97-S112. [DOI] [PubMed] [Google Scholar]
- 27. Harbison R, Hecht M, MacLeod J. Building a data-driven multiple daily insulin therapy model using smart insulin pens. J Diabetes Sci Technol. 2022;16(3):610-616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Greenwood DA, Howell F, Scher L, et al. A framework for optimizing technology-enabled diabetes and cardiometabolic care and education: the role of the diabetes care and education specialist. Diabetes Educ. 2020;46(4):315-322. [DOI] [PubMed] [Google Scholar]
- 29. MacLeod J, Scher L, Greenwood D, et al. Technology disparities and therapeutic inertia—a call to action for the diabetes care and education specialist. ADCES Pract. 2021;9(5):34-41. [Google Scholar]
- 30. Kompala T, Neinstein AB. Smart insulin pens: advancing digital transformation and a connected diabetes care ecosystem. J Diabetes Sci Technol. 2022;16(3):596-604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Vigersky RA, Cordero TL, MacLeod J, et al. Artificial intelligence: the next frontier in diabetes therapy. Nature Milestones in Diabetes. June 17, 2021. [Google Scholar]
- 32. Anirban R, Grosman B, Engel T, Miller D, et al. Eliminating advanced meal bolusing: a feasability trial using MiniMed™ 780G and hand gesture algorithms. Oral Presentation at the American Diabetes Association 82nd Scientific Sessions; 2022; San Diego, CA. [Google Scholar]
- 33. Ospelt E, Rioles N, Scott M, et al. Identifying and addressing barriers to smart insulin pen use: a T1D exchange qualitative study of diabetes providers. Clinical Diabetes. DOI: 10.2337/cd22-0068. [DOI]
- 34. Warshaw H, Isaacs D, MacLeod J. Reference guide to integrate smart insulin pens into data-driven diabetes care and education services. Diabetes Educ. 2020;46(suppl 4):3S-20S. [DOI] [PubMed] [Google Scholar]
- 35. Smith M, Gaetano A, Thanasekaran S, et al. Smart insulin pens uncover need for long-acting insulin dose optimization in multiple daily insulin therapy. Endocr Prac. 2021;27(suppl 6):S49-S50. [Google Scholar]
- 36. Jendle J, Ericsson A, Gundgaard J, Moller JB, Valentine WJ, Hunt B. Smart insulin pens are associated with improved clinical outcomes at lower cost versus standard-of-care treatment of type 1 diabetes in Sweden: a cost effectiveness analysis. Diabetes Ther. 2021;12(1):373-388. [DOI] [PMC free article] [PubMed] [Google Scholar]