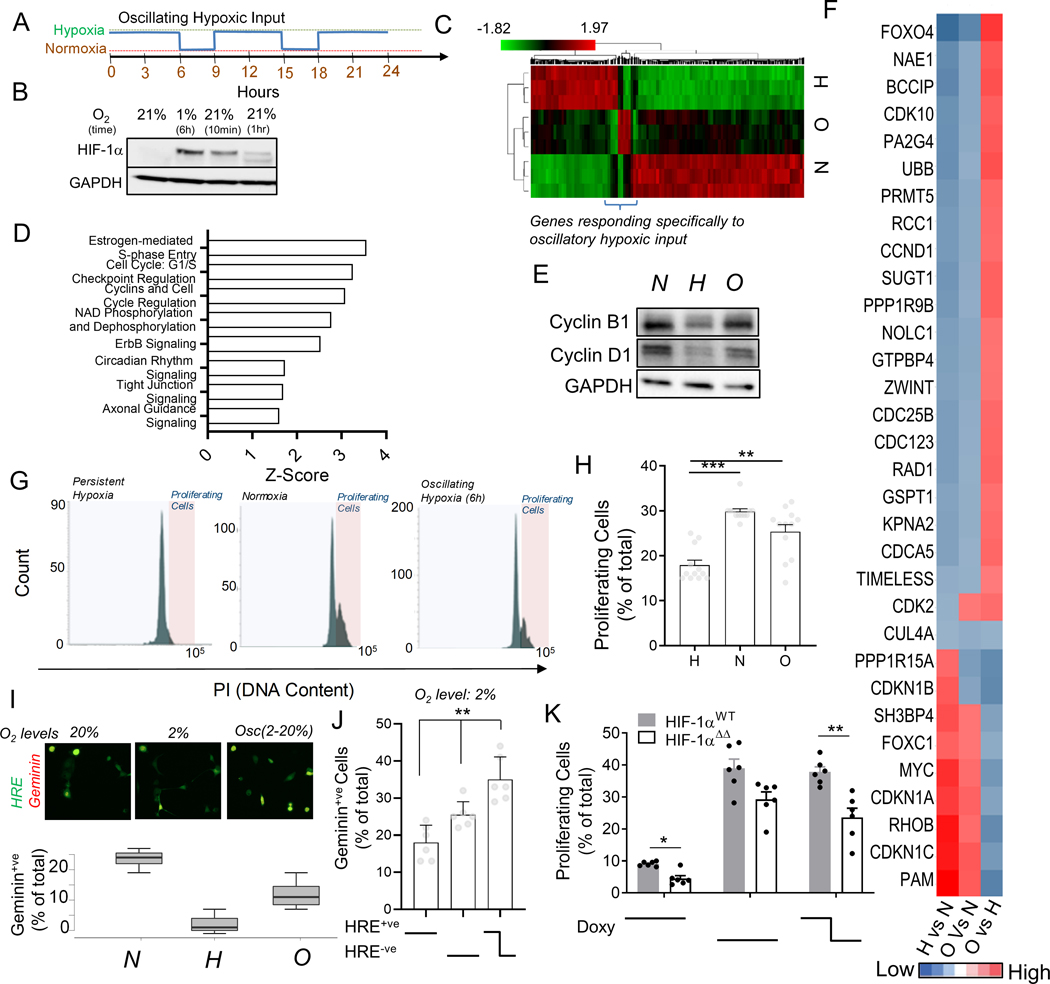
Fig 5. Oscillating hypoxic response can lead to differential gene regulation and allow proliferation under hypoxia.
(A) Experimental protocol to entrain intracellular hypoxic response by imposing oscillations of extracellular O2. (B) Immunoblot showing HIF-1α abundance in HeLa cells after 1% hypoxia for 6 hours, and after re-oxygenation for 10 min, and 1 hour, GAPDH is loading control; (C) RNA-Seq based hierarchical clustering of gene expression in HeLa cells kept in normoxia, and stable or oscillating hypoxia for > 24 h; Genes differentially regulated by oscillatory hypoxia shown in bracket. (See Fig S5). (D) Key Ingenuity pathways upregulated in gene-set differentially regulated by oscillatory hypoxia (referred to in B) vs persistent hypoxia. (E-J) Oscillations in HIF-1α abundance allows cells to continue to proliferate in hypoxia; (E) Immunoblot showing abundance of Cyclin B1 and Cyclin D1 in cells in normoxia, and stable or oscillating hypoxia; GAPDH is loading control. (F) RNA-Seq-based analysis of pairwise fold-changes between cells in above conditions for cell-cycle related genes. (G) Propidium iodide levels in HEK293 cells after conditioning with stably high, low or oscillatory input of 2% oxygen for 18 h; Quantification of gated cycling cells shown in (H); n = 12 exp. (I) Percentage of HypoxCR-GFP transfected HeLa cells positive for Geminin-mCherry after 12 h each of environmental normoxia, hypoxia, and oscillating hypoxia; above panel shows a representative location. (J) Percentage of HypoxCR-GFP transfected HEK293 cells positive for Geminin-mCherry within HRE-GFP+ve, HRE-GFP-ve subpopulations, and cells that spontaneously oscillate in persistent hypoxia; n = 6 exp. (K) Percentage of proliferating cells in HeLa cells stably transfected with Tet-inducible wild-type HIF-1α (HIF-1αWT) and HIF-1α with mutation in KFERQ-like lysosomal targeting motif (HIF-1αΔΔ), with stable or oscillating doxycycline concentrations; n = 6 exp. Error bars: s.e.m.; *: p < 0.05, **: p < 0.01, ***: p < 0.001.