Figure 1.
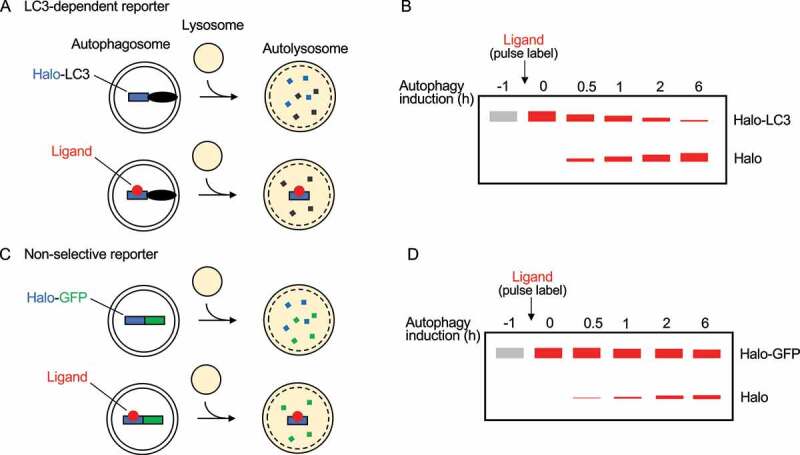
The HaloTag processing assay. (A) Halo-LC3 is completely degraded in autolysosomes but pulse-labeled Haloligand-LC3 releases free Haloligand when processed by lysosomal enzymes. (B) Haloligand-LC3 processing is observed by SDS-PAGE methods, namely immunoblotting and in-gel fluorescence imaging. Along with a decrease in Haloligand-LC3 protein levels, the amount of free Haloligand produced after autophagy induction increases over time, indicating that it is a clear indicator of autophagic flux. (C) As described in (A), pulse-labeling the bulk non-selective reporter Halo-GFP is required for free Haloligand to persist in autolysosomes after the reporter has been processed in autolysosomes. (D) As described in (B), Haloligand-GFP processing is detected by SDS-PAGE methods. The decrease in Haloligand-GFP protein levels over time is not as obvious as Halo-LC3 because Haloligand-GFP is a cytosolic reporter that is randomly incorporated into phagophores. However, bulk nonselective autophagy can still be readily detected by the production of free Haloligand.
