Fig. 10.
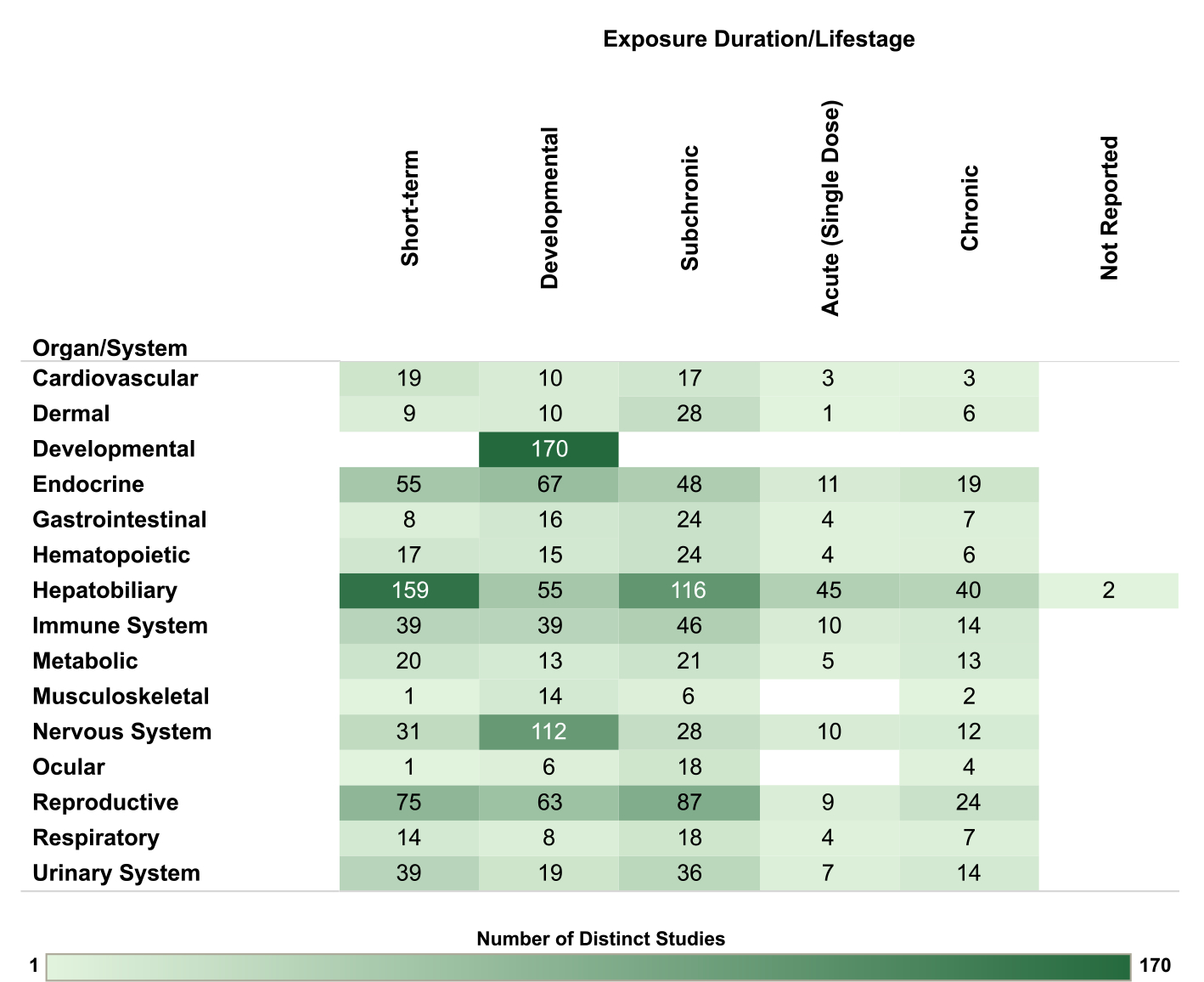
Overview of Nonhuman Mammalian Studies by Organ/System and Exposure Duration/Lifestage. Summary of the database of studies in nonhuman mammals evaluating exposures to PCB mixtures and health endpoints organized by system and exposure duration/lifestage. Lists of studies included in each count can be accessed via the online interactive version of this figure (https://hawc.epa.gov/summary/visual/assessment/100500282/OverviewNonh umanMammalStudies/). The online figure can be expanded to include information by endpoint category and can be filtered by organ/system (options: cardiovascular, dermal, developmental, endocrine, gastrointestinal, hematopoietic, hepatobiliary, immune system, metabolic, musculoskeletal, nervous system, ocular, reproductive, respiratory, urinary system), exposure duration/life stage (options: acute [single dose], chronic, developmental, NR, short-term, subchronic), species (options: cat, cow, dog, ferret, gerbil, goat, guinea pig, hamster, mink, mouse, nonhuman primate, rabbit, rat, sheep, swine, vole), sex (relevant only for reproductive endpoints; options: female, male, pair), and exposure route (options: dermal, inhalation, injection, oral). Shading intensity corresponds with the number of studies in each category, from 1 to 170, which is the maximum number of studies in any category. NR = not reported.
