Fig. 8.
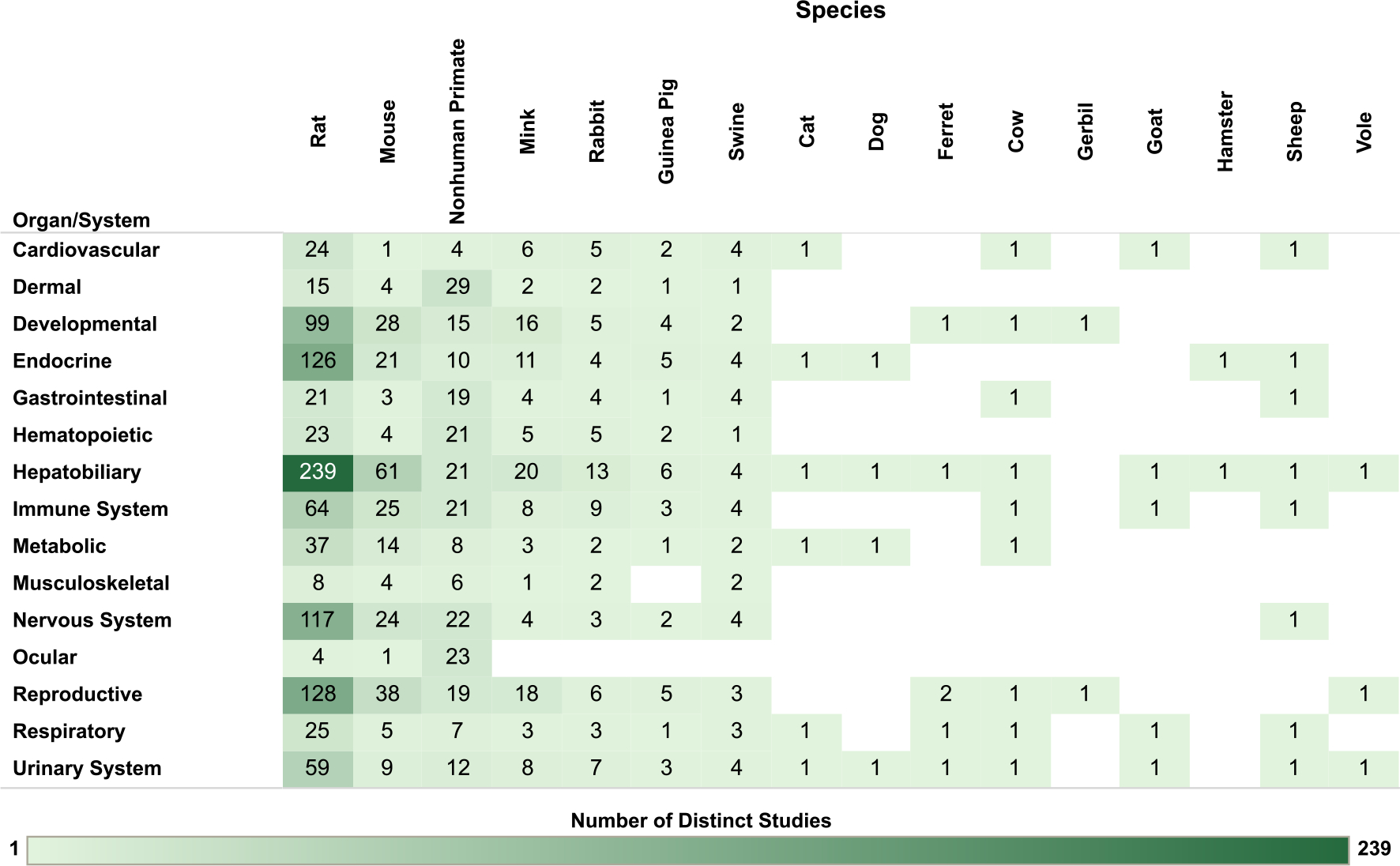
Overview of Nonhuman Mammalian Studies by Organ/System and Species. Summary of the database of studies in nonhuman mammals evaluating exposures to PCB mixtures and health endpoints organized by system and species. Lists of studies included in each count can be accessed via the online interactive version of this figure (https://hawc.epa.gov/summary/visual/assessment/100500282/OverviewNonhumanMammalStudies/). The online figure can be expanded to include information by endpoint category and can be filtered by organ/system (options: cardiovascular, dermal, developmental, endocrine, gastrointestinal, hematopoietic, hepatobiliary, immune system, metabolic, musculoskeletal, nervous system, ocular, reproductive, respiratory, urinary system), exposure duration/life stage (options: acute [single dose], chronic, developmental, NR, short-term, subchronic), species (options: cat, cow, dog, ferret, gerbil, goat, guinea pig, hamster, mink, mouse, nonhuman primate, rabbit, rat, sheep, swine, vole), sex (relevant only for reproductive endpoints; options: female, male, pair), and exposure route (options: dermal, inhalation, injection, oral). Shading intensity corresponds with the number of studies in each category, from 1 to 239, which is the maximum number of studies in any category. NR = not reported.
