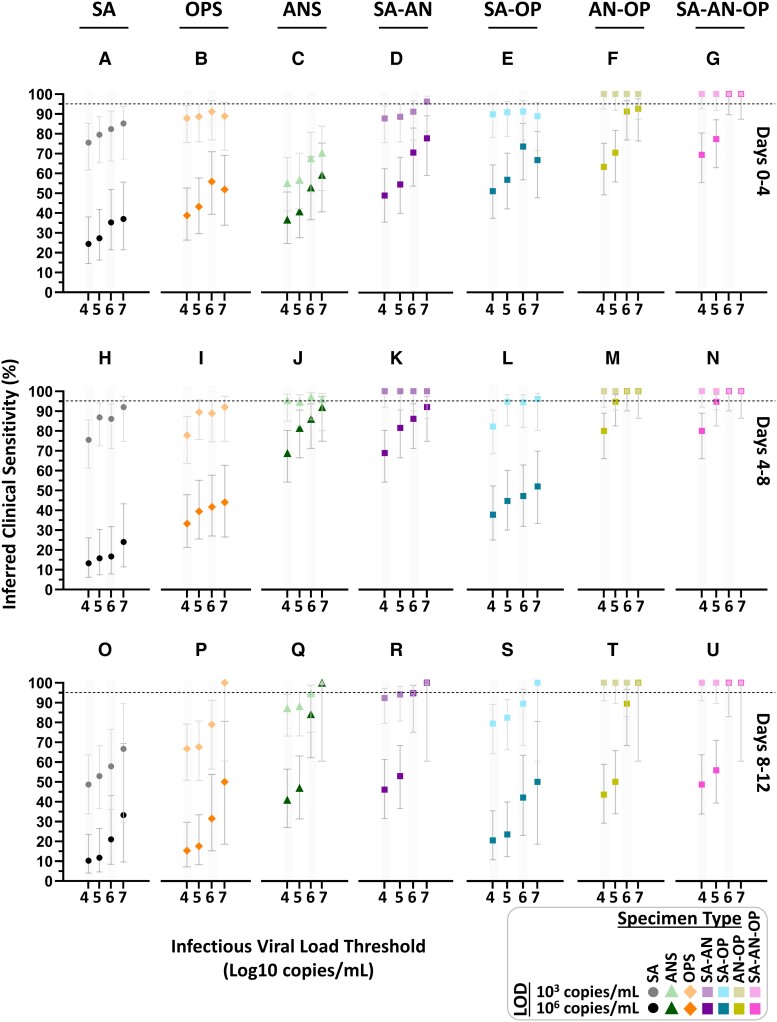
Fig. 6.
Inferred clinical sensitivity of high- and low-analytical-sensitivity assays to detect presumed infectious individuals by testing single and combination specimen types throughout acute, incident infection. For each 4-day timebin (A–G, H–N, and O–U) relative to the first SARS-CoV-2 positive specimen (of any type), participants were classified as being presumed infectious if viral load in any specimen type collected at a given timepoint was above an infectious viral load threshold. For a high-analytical-sensitivity assay with an LOD of 103 copies/mL and low-analytical-sensitivity assay with an LOD of 106 copies/mL, the inferred clinical sensitivity was calculated as the number of specimens of that specimen type with a measured viral load at or above the LOD divided by the total specimen-collection timepoints included in that timebin. Error bars indicate the 95% CI. The viral load of computationally contrived combination specimen types was taken as the higher viral load of the specimen types included in the combination collected by a participant at a given timepoint. SA, saliva; ANS, anterior-nares swab; OPS, oropharyngeal (throat) swab; SA–AN, saliva-anterior-nares swab combination; SA-OP, saliva–oropharyngeal combination swab; AN–OPS, anterior-nares–oropharyngeal combination swab; SA–AN–OP, saliva-anterior-nares–oropharyngeal combination swab. Inferred clinical sensitivity for LODs from 102.4 to 108 copies/mL shown in Fig. S8; 2-day timebins are shown in Fig. S9.