Abstract
Background
Patients with human epidermal growth factor receptor 2-positive (HER2-positive) cancers have a high incidence of central nervous system (CNS) spread, but unfortunately systemic trastuzumab which targets the HER2 receptor has little CNS penetration. The purpose of this study was to determine the maximum-tolerated dose of intrathecal trastuzumab and its efficacy in patients with HER2-positive leptomeningeal disease (LMD).
Methods
This multicenter study enrolled 34 LMD patients in a combined phase I/II study in treating patients with intrathecal trastuzumab. Any HER2-positive histology was allowed in the phase I; the phase II was limited to HER2-positive breast cancer.
Results
Intrathecal trastuzumab was well-tolerated, with one dose limiting toxicity of grade 4 (arachnoiditis) occurring at the 80 mg twice weekly dose. The recommended phase II dose was 80 mg intrathecally twice weekly. Twenty-six patients at dose level 80 mg were included in evaluation for efficacy: partial response was seen in 5 (19.2%) patients, stable disease was observed in 13 (50.0%), and 8 (30.8%) of the patients had progressive disease. Median overall survival (OS) for phase II dose treated patients was 8.3 months (95% CI 5.2–19.6). The phase II HER2-positive breast cancer patients median OS was 10.5 months (95% CI 5.2–20.9). Pharmacokinetic (PK) studies were limited in the setting of concurrent systemic trastuzumab administration, however, did show stable cerebrospinal fluid (CSF) concentrations with repeated dosing suggest that trastuzumab does not accumulate in the CSF in toxic concentrations.
Conclusion
This study suggests promise for potentially improved outcomes of HER-positive LMD patients when treated with intrathecal trastuzumab while remaining safe and well-tolerated for patients.
Keywords: CNS metastases, HER2-positive metastases, leptomeningeal disease
Key Points.
This is the largest prospective study of patients with human epidermal growth factor receptor 2-positive (HER2-positive) leptomeningeal disease (LMD) treated with intrathecal trastuzumab.
Safety and efficacy are reported showing promise for treatment of these LMD patients with intrathecal trastuzumab.
Importance of the Study.
This original research is the largest prospective study of patients with human epidermal growth factor receptor 2-positive (HER2-positive) leptomeningeal disease (LMD) treated with intrathecal trastuzumab and includes both the phase I and phase II of this clinical trial along with critical pharmacokinetic data with intrathecal trastuzumab drug administration. In this study, the safety profile and maximum-tolerated dose of intrathecal trastuzumab was determined followed by the phase II which revealed the potential clinical benefit to this treatment and demonstrated potential improved survival outcome data as compared to historical controls. The pharmacokinetics was analyzed as a correlative assessment in this study. Understanding clinical benefit and gaining insight into the pharmacokinetics of this drug will not only help patients being treated for HER2-postive LMD, but also provides an example on conducting effective clinical research with crucial correlative studies, in this case cerebrospinal fluid (CSF) pharmacokinetics, to extract maximal scientific understanding and advancement.
Background
Leptomeningeal metastasis (LM) is a serious and life-threatening complication of many cancers. While the exact incidence is unknown, it is estimated to affect 1%–8% of all breast cancer patients, potentially the highest incidence rate among solid tumors.1–3 Overexpression of HER2 is observed in 25%–30% of patients with breast cancers.4 Throughout the course of their disease, 30%–55% of women with human epidermal growth factor receptor 2 (HER2, also known as erbB2, neu, and p185HER2)-positive breast cancer will develop central nervous system (CNS) metastases, often in the setting of well-controlled systemic disease.5–8 Among breast cancer patients, risk factors for the development of LM include node positive disease, tumors >2 cm, tumor grade 3 or higher, age less than 35 years, and HER2-positive subtype.9 As a result of promising preclinical data in breast cancer, trastuzumab, a monoclonal antibody that targets the HER2 receptor, was tested in the clinic both as a single agent and in combination with chemotherapy. Its ability to delay progression in HER2+ breast cancer and improve overall survival (OS) led to its approval in the first line setting in the United States in 1998 and in Europe in 2000 in HER2-positive breast cancer patients. HER2 positivity has also been seen in other malignancies such as esophageal cancer and other gastrointestinal cancers10 as well as gliomas,11 which has HER2-directed therapeutic implications.
It has been well-established that HER2-positive breast cancer patients on trastuzumab can often develop relapsed disease within the CNS. One study found that 34% of patients on trastuzumab developed CNS disease a median of 6 months from the onset of treatment, with half of the patients having stable or responding systemic disease; of the patients with CNS disease, 19% had LM.12 An aggregate of all trials suggests a 23%–48% rate of CNS involvement with a median time to development of 4–12 months.9,13 Reasons for CNS relapse with stable or responding systemic disease likely include the inability of trastuzumab to penetrate the CNS due to its size (145 kDa). Pestalozzi and Brignoli report a 300-fold difference in concentrations between serum and cerebrospinal fluid (CSF) in patients when it is given as a weekly intravenous regimen.14
Given prior success with systemically administered trastuzumab and the potential to achieve better drug bioavailability within the leptomeningeal space after intrathecal administration, this prospective phase I/II study was designed to evaluate the safety, optimal dosing, and early efficacy of intrathecal trastuzumab in patients with HER2-positive cancer with LM. A secondary purpose of the present study was to determine the pharmacokinetics of intrathecally administered trastuzumab.
Methods
All patients were required to sign an IRB approved informed consent in this multiinstitutional study (NCT01325207). The phase I component consisted of a modified 3 + 3 dose escalation study. Patients were enrolled on clinical trial from July 2011 to June 2016. At the time of the study design, there existed only a few case reports and case series of patients treated with intrathecal trastuzumab.15–17 Each of these anecdotal treatment regimens used varying doses of intrathecal trastuzumab at times in combination with systemic therapies. Based on this data, we chose to treat patients starting at a modest dose of intrathecal trastuzumab 10 mg twice weekly and escalate to a maximum dose of 80 mg intrathecally twice weekly. After cohorts 1 and 2, an accelerated phase I study was conducted for cohorts 3 and 4, followed by a standard 3 + 3 study for cohort 5. In the accelerated component, 1 patient was enrolled per cohort; if a toxicity was seen in that patient, then the cohort would be expanded to 6 patients to allow for 1/6 patients per cohort to have a dose limiting toxicity before dose escalation. Cohort 5 enrolled a total of 6 patients. Intrathecal dosing was as follows: cohort 1—10 mg, cohort 2—20 mg, cohort 3—40 mg, cohort 4—60 mg, and cohort 5—80 mg. Patients were treated twice weekly for 4 weeks, then once a week for 4 weeks, and then every 2 weeks. Dose limiting toxicity was assessed during the first 4 weeks of treatment. Electrocardiogram testing was done prior to registration at baseline and day 1 of each subsequent cycle. CTCAEv4.03 was used in reporting adverse events (AEs). Given that trastuzumab is an FDA approved drug, there were no predefined dose limiting toxicities outlined. Expected AEs were defined as those events listed in the package insert or within the investigational brochure and unexpected AEs were those not listed within these two entities.
The phase I component allowed for any locally tested HER2-positive systemic cancer histology as well as primary tumors (medulloblastoma, glioblastoma, and ependymoma). There was no limit on prior systemic or intrathecal chemotherapies and concurrent systemic trastuzumab was allowed as were non-CNS penetrating systemic therapies. Patients were required to be at least 18 years of age, able to undergo informed consent, have a Karnofsky Performance Status (KPS) of at least 50 and must have had CSF cytology confirmation of leptomeningeal disease (LMD) if not seen radiographically. Exclusion criteria included patients who required concurrent whole brain radiation therapy (WBRT) or any other concurrent radiation therapy (with the exception of palliative radiotherapy to a localized region in the spine for pain control, ie, vertebral disease, pelvic bony disease). Patients with any contraindication for Ommaya reservoir placement were also excluded. Left ventricular ejection fraction (LVEF) of at least 45% was required along with a life expectancy of at least 8 weeks. There were no limitations on number of prior systemic or intrathecal therapies.
The phase II component of this study aimed to determine the radiological, cytological, and clinical responses to intrathecal trastuzumab. As no validated measure of LM response exists, the response was assessed using a combination of three components. The components consisted of clinical, radiographic (MRI brain and whole spine), and CSF cytology responses, as outlined below in Table 1. MRI of the brain and spine was done 4 weeks (after cycle 1) and again after cycle 2 (after 8 weeks) with concurrent clinical and cytologic evaluation. After cycle 2, these same three assessments (imaging, cytologic, and clinical) were completed at least every 6–8 weeks (typically prior to every odd numbered cycle). The phase II study was conducted with the phase I determined maximum-tolerated dose in patients with a HER2-positive breast cancer histology. For phase II, a 2-stage Simon phase II optimal design was used with a Type I error rate of 10% and a Type II error rate of 10%. Nine patients were to be observed in the first stage, with at least one response needed to expand the phase II component. If this threshold was met, an additional 15 patients were to be enrolled. The actual response rate was estimated using 95% confidence interval methods for two-stage trials using all patients evaluable for response. Response was assessed with MRI brain and spine done on study prior to cycle 1 and cycle 2 and thereafter of every odd cycle. One cycle was defined as 28 days. CSF cytology was done on day 1 and 15 of cycle 1 for phase I patients. For phase II patients, CSF cytology was completed on day 1 and 15 of cycles 1 and 2. Thereafter CSF cytology was done on day 1 of each cycle. Neurological exams were assessed on day 1 and day 15 during cycle 1, and then day 1 of each subsequent cycle. The phase II component of this study exclusively included HER2-positive breast cancer patients in order to limit outcome variability that other primary histology could produce.
Table 1.
Response assessment for leptomeningeal metastases as defined on protocol
| Complete response (CR) = complete clinical response (CCR) + radiographic complete response (RCR) and stable or improved clinical function |
| Partial response (PR) = CCR with stable imaging AND stable or improved clinical function OR |
| Partial response (PR) = RCR + stable cytology AND stable or improving clinical function |
| Stable disease (SD) = no change in cytology or imaging with stable clinical function |
| Progressive disease (PD) = progression in cytology, clinical function and radiographic findings |
The pharmacokinetics of the intrathecally administered trastuzumab dose was determined in patients in phase I. Matched CSF and venous blood samples were to be obtained immediately before and 1, 2, 4, 6, 8, and 24 h after administering dose 1 in week 1. All CSF samples were obtained via Ommaya port. Patients were asked to participate in additional sample collection 48 h post dose. They were also asked to participate in additional optional collection immediately before administration of dose 2 in week 1 and 1–2 h after dose administration as well as immediately before and 1–2 h after administering doses 3 through 8 in weeks 2 through 4.
In terms of actual trastuzumab administrations, each dose of trastuzumab was administered IT via Ommaya reservoir over 2–5 min and 2–3cc of CSF was removed prior to trastuzumab administration to flush the butterfly line after IT trastuzumab. If the Ommaya had be removed during treatment for any reason, trastuzumab was allowed to be administered via lumbar puncture after day 3 of treatment and until a new one could be placed (although this did not occur throughout the course of the clinical trial). Treatment was held for patients whose platelets were <50 000 or ANC < 1.0.
CSF and serum trastuzumab concentrations were measured at Covance Laboratories (Chantilly, VA) using an enzyme linked immunosorbent assay (ELISA). The lower limits of quantitation for trastuzumab were 20 ng/mL for CSF and 156 ng/mL for serum. The CSF pharmacokinetics were characterized by compartmental pharmacokinetic analysis. CSF trastuzumab concentration versus time relationships were modeled using the SAAM II software system (SAAM Institute, Seattle, WA) implemented on a Windows-based personal computer in those patients from whom at least four of the samples scheduled to be obtained in the first 8 h after drug administration had been collected. The systemic absorption from the CSF was to be determined by a standard two-stage approach if there were sufficient serum samples with measurable trastuzumab concentrations. The CSF pharmacokinetics, including apparent volume of distribution, clearance, and half-life, were characterized by compartmental pharmacokinetic analysis.
Results
A total of 34 patients were treated with intrathecal trastuzumab in the combined phase I/II protocol from July 2011 to June 2016. Of the 34 patients, 26 were treated at the recommended phase II dose of 80 mg intrathecally (n = 2 carry over from the phase I and n = 24 from the expansion/phase II). Patient characteristics are summarized in Table 2. The median age of all patients was 51-year-old (range 25–69). Median number of cycles administered was 2 cycles (1 cycle = 4 weeks) and median follow-up time was 10.5 months. Of the 26 phase II patients, the median age was 51 years (range 25–69 years). Most patients (25/26) had previously had CNS directed therapy, and all but one patient had prior brain directed radiation. These CNS directed therapies included 13 patients with prior LM directed therapy, 13 patients with prior WBRT, 13 patients with prior stereotactic radiosurgery, and 2 patients with prior intrathecal therapy. Ten patients were on systemic directed therapy concurrently at the time of intrathecal therapy treatment and 18 patients had concurrent brain parenchymal metastases.
Table 2.
Baseline phase II patient demographic and clinical characteristics
| Characteristic (n = 26) | Range or percent of total n | |
|---|---|---|
| Median age | 51.0 | Range (25–69) |
| Median number of cycles (1 cycle = 4 weeks) | 2 | Range (1–22) |
| Median follow-up | 10.5 months | Range (0.8–39.6) |
| Her2+ Bbeast cancer | 23 | 88% |
| Prior intrathecal therapy | 2 | 8% |
| Prior WBRT | 13 | 50% |
| Prior SRS | 13 | 50% |
| Concurrent brain mets | 18 | 69% |
| Systemic directed therapy at registration | 10 | 38% |
| New concurrent systemic therapy | 5 | 19% |
| Previously treated for LM | 13 | 50% |
As the phase I component was a modified 3 + 3 design, 3 patients were enrolled in the initial 10 mg intrathecal trastuzumab cohort, 3 patients in the 20 mg cohort, and one patient each in the 40 and 60 mg cohorts. At the highest planned dose, 80 mg twice weekly, one dose limiting toxicity, a grade 4 arachnoiditis, was seen and deemed related to intrathecal trastuzumab. After this grade 4 toxicity was found, an additional 3 patients were enrolled at the same 80 mg dose level. Only 1 of the 6 patients in cohort 5 (the 80 mg dose cohort), experienced this same dose limiting toxicity of grade 4 arachnoiditis at cycle 1. The Robert H. Lurie Comprehensive Cancer Center Data Monitoring Committee reviewed all of the phase I safety data and agreed that the maximum-tolerated dose should be 80 mg intrathecally as well as the recommended phase II dose. The more commonly seen side effects in patients treated with intrathecal trastuzumab were grade 1 headache in 5 treated patients (15%), at least a grade 1 of nausea in 5 patients (15%), grade 1 emesis in 3 patients (9%), and meningismus in 2 patients (6%). There were no Ommaya specific AEs to report. Twenty-six patients were then treated with the recommended phase II dose of 80 mg trastuzumab intrathecally and were evaluable for the primary endpoint of the phase II study, disease response. Treatment-related AEs and serious AEs are tabulated (Tables 3 and 4).
Table 3.
Combined results of phase 1/2 adverse events, at least possibly related to study treatment
| Toxicity | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
|---|---|---|---|---|
| Noninfectious meningitis/arachnoiditis | 1 | 2 | 1 | |
| Headache | 3 | |||
| Fatigue | 1 | 1 | ||
| Laryngitis | 1 | |||
| Fever | 1 | |||
| Nausea | 1 | |||
| Malaise | 1 | |||
| Vertigo | 1 | |||
| Anorexia | 1 |
Table 4.
Combined results of phase 1/2 serious adverse events, deemed related to study treatment
| Toxicity | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
|---|---|---|---|---|
| Noninfectious meningitis/arachnoiditis | 1 | 1 | ||
| Hydrocephalus | 1 | |||
| Nausea | 1 | |||
| Headache | 2 | |||
| Vomiting | 1 | |||
| Back pain | 1 | |||
| Extremity pain | 1 |
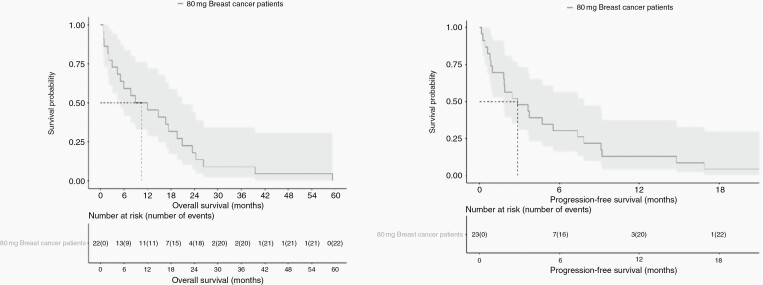
Table 2 highlights the key characteristics for patients enrolled in the phase II study. As there is not a validated measure for disease response in LM, we utilized a response scale integrating the three diagnostic components: clinical response, cytologic response, and radiographic response (Table 1). As the defined statistical target for expansion was met, the study continued to complete enrollment of 26 patients. The best response seen in these 26 patients was: partial response in 5 patients (19.2%), stable disease in 13 patients (50.0%), and progressive disease in 8 patients (30.8%), making the clinical benefit/disease control rate 69.2% defined as stable disease or better. Median progression free survival (PFS) for the 26 patients in the phase II study was 2.2 months (95% CI 1.0–7.4) and median OS was 8.3 months (95% CI 5.2–19.6). Of these 26 patients, 23 carried a diagnosis of HER2-positive breast cancer and the remaining 3 had HER2-positive solid tumors of other histologies (1 lung adenocarcinoma, 1 colorectal cancer, and 1 anaplastic ependymoma). In the HER2-positive breast cancer patient subset, the median PFS was 2.8 months (95% CI 1.8–7.8) and median OS was 10.5 months (95% CI 5.2–20.9). Figures 1 and 2 demonstrate Kaplan–Meier curves of PFS and OS. The specific response in each domain per patient is outlined in detail in the supplementary table given the complexity of response in LMD and the lack of one central-validated response measure.
Fig. 1.
(a and b) Progression free survival (PFS) and Overall Survival (OS) of the 23 HER2-positive breast cancer leptomeningeal disease patients treated with 80 mg intrathecal doses of trastuzumab (median PFS = 2.8 months, median OS= 10.5 months).
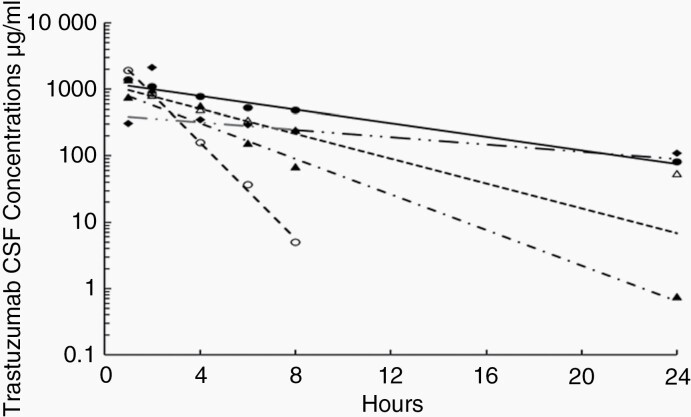
Fig. 2.
Representative fits of the pharmacokinetic model to the cerebrospinal fluid (CSF) trastuzumab concentrations measured up to 24 h after administration of 80 mg intrathecally to 5 patients. The symbols represent measured drug concentrations while the lines represent the fits of the models to the data.
Ten patients (two after administration of a 10 mg dose, two after a 20 mg dose, one after a 60 mg dose, and five after an 80 mg dose) had CSF concentrations measured in at least four of the samples scheduled to be obtained in the first 8 h after administration of the first intrathecal dose of trastuzumab to allow meaningful analysis. The CSF pharmacokinetics of trastuzumab was well characterized in these patients by a one-compartment pharmacokinetic model. Representative fits of the pharmacokinetic model to CSF trastuzumab concentration versus time relationships of the 5 patients to whom 80 mg of trastuzumab was administered intrathecally are illustrated in Figure 2. The volume of distribution (mean ± S.D) was 73 ± 48 mL, the CSF clearance was 14 ± 5 mL/h, and the apparent CSF half-life was 4.1 ± 3.0 h. There were too few serum samples collected after administration of the first intrathecal dose that had trastuzumab concentrations above the limit of detection (156 ng/mL) to enable characterization of systemic absorption from the CSF after administration of the first dose.
CSF samples were obtained before and 1–2 h after administration of intrathecal doses 3–8 in 3 of the patients receiving 80 mg trastuzumab doses. All but two of the concentrations measured in samples obtained before administration of these doses were less than 10 μg/mL. The average concentrations measured 1–2 h after these doses ranged from 1.2 to 2.2 mg/mL, which are largely consistent with the average concentration observed in the same patients 1 h after administration of dose 1, 1.4 mg/mL.
Serum trastuzumab concentrations were always measurable before administration of doses after the first dose, suggesting very delayed systemic absorption. The concentrations also tended to be slightly higher before each subsequent dose, suggesting slight accumulation due to the low systemic trastuzumab clearance.
Discussion
We conducted a phase I/II study of intrathecal trastuzumab in patients with HER2+ LM. Overall, intrathecal trastuzumab was well-tolerated and the recommended phase II dose was 80 mg intrathecally beginning twice a week followed by a tapering of frequency. The dosing schedule was 80 mg twice weekly for 4 weeks, followed by weekly for 4 weeks and then once every 1–2 weeks for maintenance therapy. The median OS across all dose ranges and histologies was 8.76 months. At the recommended phase II dose of 80 mg, the response rate (19.2% for overall population, 17.9% for HER2+ breast cancer population) and OS (of 8.34 months for the overall population, 10.5 months for the HER2+ breast cancer population) from the time of study entry reported in this study was favorable compared to established historical controls (3.3–4.4 months) for the HER2-positive breast cancer population with LM.18–20 The trend toward longer survival in the HER2+ breast cancer population may reflect a better natural history compared to LM from other HER2+ histologies.
One striking finding when evaluating the PFS and OS in our phase II study was the distinct gap between the 2.2-month median PFS and the 8.3-month median OS in the entire cohort of the 26 HER2-postive cancer patients with LM. The same discrepancy was seen in the 23 HER2-positive breast cancer specific population with median PFS of 2.8 months and median OS of 10.5 months, respectively.
The favorable OS observed is of particular interest considering the short PFS (1.89 months across all doses and histologies, 2.15 months for all patients treated at 80 mg, and 2.83 months for all HER2+ breast cancer patients treated at 80 mg). Lack of a validated response assessment for LM makes it difficult to make sense of clinical, radiographic, or cytologically based endpoints. As the OS in patients with LM is often driven by LM as opposed to systemic disease, it may be rational to consider OS as the clinical trial endpoint for defining efficacy in this disease state.
Given that there was not a control arm in our study, the best comparator for OS in this population comes from historical controls. One large contemporary study published in 2014 looked at survival of all LM patients within a specified time period and reported the median OS of breast HER2-positive LM as 4.4 months.18 Another study reported similar median OS of 3.3 months for both HER2-positive and HER2-negative breast cancer LM patients.19 Both studies reported OS from time of initial LM diagnosis, unlike our study which reported it from study enrollment, in turn, shortening the time interval. Our observed median OS for HER2-postive breast cancer LM patients in this study was favorable at 10.5 months. It can be argued that LM patients that enroll on clinical trials have favorable performance scores and may not be generalizable to the overall LM population. Furthermore, the inclusion of concomitant systemic therapy could also impact these results. At least 4 of the patients on study had systemic therapy that could be considered CNS penetrating (3 patients on lapatinib, 1 on high dose systemic methotrexate) which could be a shortcoming to our study that could impact results. It could be beneficial in future studies to evaluate neurologic versus nonneurologic cause of death to better understand the impact of CNS directed therapy and even consider as a study endpoint. In the future, we would suggest more frequent concurrently timed comparators of LMD diagnostics, including CSF cytology, CSF circulating tumor cells, and CSF cell free DNA along with clinical and radiographic findings to better capture response rate in a setting of no current longitudinally validated measure for response rate. Other clinical shortcomings included not having complete data on systemic PFS, response, pattern of recurrence, and having subsequent therapies after patients came off study therapy available only for a subset of patients. In future studies, not only do the authors suggest including these elements, but also more novel diagnostic tools such as circulating tumor DNA and circulating tumor cells within the CSF. A subset of patients within this clinical trial underwent these evaluations as substudy reported on previously.21 Nonetheless, a near tripling of median OS in the present study compared to that of recently reported survival in similar patients remains attractive for further investigation. It can also be argued that patients in our phase I/II intrathecal trastuzumab study were largely previously treated for both LM and brain parenchymal metastases (prior WBRT n = 13, prior stereotactic radiosurgery n = 13, prior intrathecal therapy n = 2) and represent a recurrent CNS metastases population who would have less favorable outcomes.
CSF penetration of systemically administered trastuzumab has been previously evaluated showing overall poor CSF bioavailability.22 The CSF pharmacokinetics of the first intrathecally administered trastuzumab dose in the present study was well characterized in patients from whom at least 4 samples were obtained in the first 8 h after administration of dose 1 (Figure 2) despite CSF not being well mixed in the classical sense (eg, as it is after intravenous administration). Although the CSF may not be well mixed, the study of another intraventricularly administered antibody, rituximab, by Rubenstein et al. found rapid craniospinal axis distribution with a rapid decline of CSF concentrations similar to the decline of trastuzumab concentrations observed in the present study.23 The observed half-life of trastuzumab in the CSF (4.1 h) cannot be assumed to be a CSF elimination half-life because the data from the present study did not lend themselves to more complex characterization. For example, Rubenstein et al. reported a multicompartmental model with a very prolonged elimination half-life largely on the basis of CSF concentrations measured 96 h post dose, which were not available in the present study.23 The CSF half-life of the present study is considerably shorter than the 18-to-30-day elimination half-life of intravenously administered trastuzumab.24,25 The short half-life, the resulting low trough CSF concentrations before administration of repeated doses two or more days later, and the similar CSF concentrations observed after repeated dosing suggest that trastuzumab will not accumulate to toxic CSF concentrations with repeated dosing. Systemically absorbed trastuzumab does accumulate because of its low systemic elimination clearance is obvious from the slight progressive increase in trough serum concentrations after repeated intrathecal doses.
The dosing regimen selected was based on the available case reports and case series that had been done before protocol development. Since that time, further reports with alternative dosing and frequency schedules have also been proposed and tested. Our study reports the first phase II findings with intrathecal trastuzumab. A European phase I study was published in 2018 using weekly intrathecal trastuzumab dosing, with dose escalation from 30 mg to a ceiling dose of 150 mg (30, 60, 100, or 150 mg dose levels). Weekly dosing was selected in this study given the long half-life of trastuzumab versus our initial twice weekly approach (which in later cycles is extended to weekly and even later to every 1–2 weeks as maintenance therapy). It is possible that sampling CSF from the site of drug administration, the Ommaya reservoir, could potentially add complications to the analysis and interpretation of the pharmacokinetics. However, the CSF concentrations and pharmacokinetic fits do not suggest that this was a problem in this study. The safety data reported from this study were similar to our findings with minimal safety concerns in this once weekly dosing schema.26 Since this was a phase I safety study, it was not powered for efficacy and did not report response and survival findings. In this study, the 16 treated patients had serum and CSF trastuzumab concentrations measured using an ELISA, with the goal of achieving a CSF concentration of at least 30 mg/mL, which they achieved. Similar to our results, no true dose limiting toxicities were found. This study offers an alternative treatment schedule to the twice weekly dose that we studied. Weekly dosing may allow a more convenient schedule; however, its efficacy has not been compared to that of the twice weekly dosing used in the present study and potentially maintaining twice weekly dosing throughout the treatment regimen may also be clinically more beneficial and needs to be further explored in future studies. The pharmacokinetic studies of our phase I/II trial support the twice weekly dosing.
Conclusion
Our phase I/II study showed that intrathecal trastuzumab was well-tolerated at an intrathecal dose of up to 80 mg dosed twice weekly as initial therapy. Pharmacokinetic studies demonstrated fairly rapid elimination from the CSF even in the setting of systemic administration, supporting initial twice a week dosing. The median OS of 8.3 months for all patients at the phase II dose and 10.5 months in HER2-positive breast cancer patients at the phase II dose in this study was favorable compared to well accepted historical controls, which reported median OS of approximately 3–4 months. Intrathecal trastuzumab may be a viable treatment options for the often-challenging treatment of HER2-positive LMD population.
Acknowledgement
This research was supported by grants the National Institutes of Health/National Cancer Institute (P30-CA008748, P30CA013696, UG1CA189960, and P50CA221747).
Contributor Information
Priya U Kumthekar, Department of Neurology at The Feinberg School of Medicine at Northwestern University and The Malnati Brain Tumor Institute at the Robert H. Lurie Comprehensive Cancer Center of Northwestern University, Chicago, Illinois, USA.
Michael J Avram, Department of Anesthesiology, Emeritus Northwestern University Feinberg School of Medicine, Chicago, Illinois, USA.
Andrew B Lassman, Division of Neuro-Oncology, Department of Neurology, Columbia University Vagelos College of Physicians and Surgeons, Herbert Irving Comprehensive Cancer Center, NewYork-Presbyterian Hospital, New York, New York, USA.
Nancy U Lin, Department of Medical Oncology, Dana-Farber Cancer Institute Harvard Medical School, Boston, Massachusetts, USA.
Eudocia Lee, Department of Medical Oncology, Dana-Farber Cancer Institute Harvard Medical School, Boston, Massachusetts, USA.
Sean A Grimm, Department of Neurology, Rush University Medical Center, Chicago, Illinois, USA.
Margaret Schwartz, Department of Neurology at The Feinberg School of Medicine at Northwestern University and The Malnati Brain Tumor Institute at the Robert H. Lurie Comprehensive Cancer Center of Northwestern University, Chicago, Illinois.
Kirsten L Bell Burdett, Department of Preventive Medicine, Division of Biostatistics, Feinberg School of Medicine, Northwestern University, Chicago, Illinois, USA.
Rimas V Lukas, Department of Neurology at The Feinberg School of Medicine at Northwestern University and The Malnati Brain Tumor Institute at the Robert H. Lurie Comprehensive Cancer Center of Northwestern University, Chicago, Illinois, USA.
Karan Dixit, Department of Neurology at The Feinberg School of Medicine at Northwestern University and The Malnati Brain Tumor Institute at the Robert H. Lurie Comprehensive Cancer Center of Northwestern University, Chicago, Illinois, USA.
Isabella Perron, Department of Neurosurgery at The Feinberg School of Medicine at Northwestern University, Chicago, Illinois, USA.
Hui Zhang, Department of Preventive Medicine, Division of Biostatistics, Feinberg School of Medicine, Northwestern University, Chicago, Illinois, USA.
William J Gradishar, Department of Medicine at The Feinberg School of Medicine at Northwestern University at The Robert H. Lurie Comprehensive Cancer Center of Northwestern University, Chicago, Illinois, USA.
Elena I Pentsova, Department of Neurology, Memorial Sloan Kettering Cancer Center, New York, New York, USA.
Suriya Jeyapalan, Department of Neurology, Tufts Medical Center, Boston, Massachusetts, USA.
Morris D Groves, Texas Oncology-Austin Brain Tumor Center, Austin, Texas, USA.
Michelle Melisko, Department of Medicine at the University of California San Francisco, San Francisco, California, USA.
Jeffrey J Raizer, Department of Neurology at The Feinberg School of Medicine at Northwestern University and The Malnati Brain Tumor Institute at the Robert H. Lurie Comprehensive Cancer Center of Northwestern University, Chicago, Illinois, USA.
Conflict of interest statement
A.B.L.: In the last 24 months received research support (to the institution) from AbbVie, Novartis, Pfizer, Genentech/Roche, Millenium, Aeterna Zentaris, Kadmon, VBI Vaccines, Beigene, NextSource, DelMar, Corden, Kazia, Servier, QED, BMS, Semus, AZ, Chimerix, Bayer, Orbus; and received honoraria/consulting fees from Bayer, Bioclinica as an expert blinded independent reviewer of deidentified clinical data for a BMS-sponsored trial, Chimerix, Karyopharm, Novocure, Orbus, Sapience, and Vivacitas/Aptitude Health. P.K.: Research and grant support from Genentech, Novocure, DNAtrix and Orbus Therapeutics. P.K. has served on medical advisory boards for Biocept, Sintetica, Novocure, Janssen, Affinia, Celularity, and SDP Oncology. P.K. has provided consulting to Bliss Bio, Biocept, Enclear Therapies, Angiochem, Affinia Therapeutics. E.P.: E.P. is a study PI for Y-mAbs Therapeutic Inc. who provides clinical trial support to the institution (MSKCC): financial or/and drug supply. E.L.: Royalties from Wolters Kluwer for Up to Date; Honoraria from MedScape. N.L.: N.L. receives research support from Genentech, Merck, Pfizer, Seattle Genetics, AstraZeneca, and Zion Pharmaceuticals. N.L. receives honorarium from Pfizer, Puma, Seattle Genetics, Daiichi Sankyo, AstraZeneca, Prelude Therapeutics, Denali Therapeutics, Olema Pharmaceuticals, Aleta BioPharma, Affinia Therapeutics, and Voyager Therapeutics. N.L. receives royalties from Up-to-date. All Research Support goes to Institution.
Author Contribution
Clinical trial execution, study conduct and accrual: P.K., J.R., A.B.L., I.P., N.L., E.L., S.G., M.S., W.G., E.P., S.J., M.G., and M.M.; Manuscript editing: P.K., J.R., A.B.L., I.P., N.L., E.L., S.G., M.S.,W.G., E.P., S.J., M.G., M.M., R.L., and K.D.; Pharmakokinetic analysis: M.J.A.; Biostatistical support: K.B.B. and H.Z.
Data Availability Statement
Raw data for this study were generated at Northwestern University. Derived data supporting the findings of this study are available from the corresponding author upon request.
References
- 1. Franzoi MA, Hortobagyi GN. Leptomeningeal carcinomatosis in patients with breast cancer. Crit Rev Oncol Hematol. 2019; 135:85–94. [DOI] [PubMed] [Google Scholar]
- 2. Mittica G, Senetta R, Richiardi L, et al. . Meningeal carcinomatosis underdiagnosis and overestimation: incidence in a large consecutive and unselected population of breast cancer patients. BMC Cancer. 2015; 15:1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Boogerd W, Hart AA, van der Sande JJ, Engelsman E. Meningeal carcinomatosis in breast cancer. Prognostic factors and influence of treatment. Cancer. 1991; 67(6):1685–1695. [DOI] [PubMed] [Google Scholar]
- 4. Howlader N, Altekruse SF, Li CI, et al. . US incidence of breast cancer subtypes defined by joint hormone receptor and HER2 status. J Natl Cancer Inst. 2014; 106(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Aversa C, Rossi V, Geuna E, et al. . Metastatic breast cancer subtypes and central nervous system metastases. Breast. 2014; 23(5):623–628. [DOI] [PubMed] [Google Scholar]
- 6. Guy CT, Webster MA, Schaller M, et al. . Expression of the neu protooncogene in the mammary epithelium of transgenic mice induces metastatic disease. Proc Natl Acad Sci USA. 1992; 89(22):10578–10582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hudziak RM, Schlessinger J, Ullrich A. Increased expression of the putative growth factor receptor p185HER2 causes transformation and tumorigenesis of NIH 3T3 cells. Proc Natl Acad Sci USA. 1987; 84(20):7159–7163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pestalozzi BC, Holmes E, de Azambuja E, et al. . CNS relapses in patients with HER2-positive early breast cancer who have and have not received adjuvant trastuzumab: a retrospective substudy of the HERA trial (BIG 1-01). Lancet Oncol. 2013; 14(3):244–248. [DOI] [PubMed] [Google Scholar]
- 9. Shmueli E, Wigler N, Inbar M. Central nervous system progression among patients with metastatic breast cancer responding to trastuzumab treatment. Eur J Cancer. 2004; 40(3):379–382. [DOI] [PubMed] [Google Scholar]
- 10. Fusco N, Bosari S. HER2 aberrations and heterogeneity in cancers of the digestive system: implications for pathologists and gastroenterologists. World J Gastroenterol. 2016; 22(35):7926–7937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhang C, Burger MC, Jennewein L, et al. . ErbB2/HER2-specific NK cells for targeted therapy of glioblastoma. J Natl Cancer Inst. 2016; 108(5). [DOI] [PubMed] [Google Scholar]
- 12. Bendell JC, Domchek SM, Burstein HJ, et al. . Central nervous system metastases in women who receive trastuzumab-based therapy for metastatic breast carcinoma. Cancer. 2003; 97(12):2972–2977. [DOI] [PubMed] [Google Scholar]
- 13. Yau T, Swanton C, Chua S, et al. . Incidence, pattern and timing of brain metastases among patients with advanced breast cancer treated with trastuzumab. Acta Oncol. 2006; 45(2):196–201. [DOI] [PubMed] [Google Scholar]
- 14. Pestalozzi BC, Brignoli S. Trastuzumab in CSF. J Clin Oncol. 2000; 18(11):2349–2351. [DOI] [PubMed] [Google Scholar]
- 15. Laufman LR, Forsthoefel KF. Use of intrathecal trastuzumab in a patient with carcinomatous meningitis. Clin Breast Cancer. 2001; 2(3):235. [DOI] [PubMed] [Google Scholar]
- 16. Stemmler HJ, Mengele K, Schmitt M, et al. . Intrathecal trastuzumab (Herceptin) and methotrexate for meningeal carcinomatosis in HER2-overexpressing metastatic breast cancer: a case report. Anticancer Drugs. 2008; 19(8):832–836. [DOI] [PubMed] [Google Scholar]
- 17. Stemmler HJ, Schmitt M, Harbeck N, et al. . Application of intrathecal trastuzumab (Herceptintrade mark) for treatment of meningeal carcinomatosis in HER2-overexpressing metastatic breast cancer. Oncol Rep. 2006; 15(5):1373–1377. [DOI] [PubMed] [Google Scholar]
- 18. Abouharb S, Ensor J, Loghin ME, et al. . Leptomeningeal disease and breast cancer: the importance of tumor subtype. Breast Cancer Res Treat. 2014; 146(3):477–486. [DOI] [PubMed] [Google Scholar]
- 19. de Azevedo CR, Cruz MR, Chinen LT, et al. . Meningeal carcinomatosis in breast cancer: prognostic factors and outcome. J Neurooncol. 2011; 104(2):565–572. [DOI] [PubMed] [Google Scholar]
- 20. Kak M, Nanda R, Ramsdale EE, Lukas RV. Treatment of leptomeningeal carcinomatosis: current challenges and future opportunities. J Clin Neurosci. 2015; 22(4):632–637. [DOI] [PubMed] [Google Scholar]
- 21. Malani R, Fleisher M, Kumthekar P, et al. . Cerebrospinal fluid circulating tumor cells as a quantifiable measure of leptomeningeal metastases in patients with HER2 positive cancer. J Neurooncol. 2020; 148(3):599–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Stemmler HJ, Schmitt M, Willems A, Bernhard H, Harbeck N, Heinemann V. Ratio of trastuzumab levels in serum and cerebrospinal fluid is altered in HER2-positive breast cancer patients with brain metastases and impairment of blood-brain barrier. Anticancer Drugs. 2007; 18(1):23–28. [DOI] [PubMed] [Google Scholar]
- 23. Rubenstein JL, Fridlyand J, Abrey L, et al. . Phase I study of intraventricular administration of rituximab in patients with recurrent CNS and intraocular lymphoma. J Clin Oncol. 2007; 25(11):1350–1356. [DOI] [PubMed] [Google Scholar]
- 24. Leyland-Jones B, Gelmon K, Ayoub JP, et al. . Pharmacokinetics, safety, and efficacy of trastuzumab administered every three weeks in combination with paclitaxel. J Clin Oncol. 2003; 21(21):3965–3971. [DOI] [PubMed] [Google Scholar]
- 25. Quartino AL, Li H, Kirschbrown WP, et al. . Population pharmacokinetic and covariate analyses of intravenous trastuzumab (Herceptin((R))), a HER2-targeted monoclonal antibody, in patients with a variety of solid tumors. Cancer Chemother Pharmacol. 2019; 83(2):329–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bonneau C, Paintaud G, Tredan O, et al. . Phase I feasibility study for intrathecal administration of trastuzumab in patients with HER2 positive breast carcinomatous meningitis. Eur J Cancer. 2018; 95:75–84. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Raw data for this study were generated at Northwestern University. Derived data supporting the findings of this study are available from the corresponding author upon request.